Visualizing lipid behind the retina in aging and age-related macular degeneration, via indocyanine green angiography (ASHS-LIA)
- PMID: 35314773
- PMCID: PMC9391351
- DOI: 10.1038/s41433-022-02016-3
Visualizing lipid behind the retina in aging and age-related macular degeneration, via indocyanine green angiography (ASHS-LIA)
Abstract
Age-related macular degeneration (AMD) causes legal blindness in older adults worldwide. Soft drusen are the most extensively documented intraocular risk factor for progression to advanced AMD. A long-standing paradox in AMD pathophysiology has been the vulnerability of Asian populations to polypoidal choroidal vasculopathy (PCV) in the presence of relatively few drusen. Age-related scattered hypofluorescent spots on late phase indocyanine green angiography (ASHS-LIA) was recently proposed as precursors of PCV. Herein, we offer a resolution to the paradox by reviewing evidence that ASHS-LIA indicates the diffuse form of lipoprotein-related lipids accumulating in Bruch's membrane (BrM) throughout adulthood. Deposition of these lipids leads to soft drusen and basal linear deposit (BLinD), a thin layer of soft drusen material in AMD; Pre-BLinD is the precursor. This evidence includes: 1. Both ASHS-LIA and pre-BLinD/BLinD accumulate in older adults and start under the macula; 2. ASHS-LIA shares hypofluorescence with soft drusen, known to be physically continuous with pre-BLinD/BLinD. 3. Model system studies illuminated a mechanism for indocyanine green uptake by retinal pigment epithelium. 4. Neither ASHS-LIA nor pre-BLinD/ BLinD are visible by multimodal imaging anchored on current optical coherence tomography, as confirmed with direct clinicopathologic correlation. To contextualize ASHS-LIA, we also summarize angiographic characteristics of different drusen subtypes in AMD. As possible precursors for PCV, lipid accumulation in forms beyond soft drusen may contribute to the pathogenesis of this prevalent disease in Asia. ASHS-LIA also might help identify patients at risk for progression, of value to clinical trials for therapies targeting early or intermediate AMD.
摘要: 年龄相关性黄斑变性 (AMD) 是全球老年人致盲的主要原因之一。软性玻璃膜疣是其进展为晚期AMD最广为人知的眼内风险因素。而在AMD的病理生理学中有一个长期存在的悖论, 即在玻璃膜疣相对较少的情况下, 亚洲人群患息肉状脉络膜血管病变 (PCV) 的风险更高。我们最近的研究提出, 吲哚青绿血管造影晚期显现的年龄相关性散在低荧光点(ASHS-LIA)是PCV形成的先兆。在此, 我们回顾ASHS-LIA代表脂蛋白相关性脂质随着年龄增长在Bruch膜(BrM)中弥散性聚集的研究证据, 从而解释这一悖论。这些脂质沉积导致了软玻璃膜疣和基底线性沉积(BLinD)的形成,其中BLinD是软性玻璃膜疣样物质的弥散性沉积形式 (Pre-BLinD是其前体)。这些研究证据包括: 1、ASHS-LIA和pre-BLinD/ BLinD均在老年人眼内沉积, 并均始于黄斑下;2.ASHS-LIA与软性玻璃膜疣均表现为低荧光, 在结构上与pre-BLinD/BLinD有连续性。3.模型系统研究阐明了视网膜色素上皮摄取吲哚青绿的分子机制。4. ASHS-LIA和pre-BLinD/BLinD在包括光学相干断层扫描的多模式成像中均不可见, 而我们的直接临床病理相关性研究也证实了这一点。此外, 我们还总结了AMD中不同玻璃膜疣亚型的血管造影特征。作为PCV的可能先兆, 软玻璃膜疣以外形式的脂质沉积可能与这种亚洲人群多发疾病的发病机制有关。ASHS-LIA还可能有助于识别有进展风险的AMD患者, 对靶向治疗早期或中期AMD的临床试验有价值。.
© 2022. The Author(s), under exclusive licence to The Royal College of Ophthalmologists.
Conflict of interest statement
CAC receives research funds from Genentech/Hoffman La Roche and Regeneron.
Figures


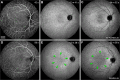
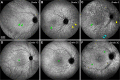
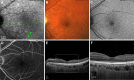
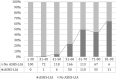

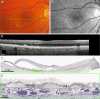
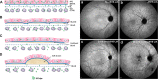


Comment in
-
Structural integrity of retinal pigment epithelial cells in eyes with age-related scattered hypofluorescent spots on late phase indocyanine green angiography (ASHS-LIA).Eye (Lond). 2023 Feb;37(2):377-378. doi: 10.1038/s41433-022-02232-x. Epub 2022 Sep 17. Eye (Lond). 2023. PMID: 36115884 Free PMC article. No abstract available.
Similar articles
-
Drusen and Age-Related Scattered Hypofluorescent Spots on Late-Phase Indocyanine Green Angiography, a Candidate Correlate of Lipid Accumulation.Invest Ophthalmol Vis Sci. 2018 Oct 1;59(12):5237-5245. doi: 10.1167/iovs.18-25124. Invest Ophthalmol Vis Sci. 2018. PMID: 30383195
-
Age-Related Scattered Hypofluorescent Spots on Late-Phase Indocyanine Green Angiography as Precursor Lesions of Polypoidal Choroidal Vasculopathy.Invest Ophthalmol Vis Sci. 2019 May 1;60(6):2102-2109. doi: 10.1167/iovs.19-26968. Invest Ophthalmol Vis Sci. 2019. PMID: 31095678
-
Age-related scattered hypofluorescent spots on late-phase indocyanine green angiography: the multimodal imaging and relevant factors.Clin Exp Ophthalmol. 2018 Nov;46(8):908-915. doi: 10.1111/ceo.13306. Epub 2018 May 10. Clin Exp Ophthalmol. 2018. PMID: 29675907 Free PMC article.
-
[Drusen in Bruch's membrane. Their significance for the pathogenesis and therapy of age-associated macular degeneration].Ophthalmologe. 1992 Oct;89(5):363-86. Ophthalmologe. 1992. PMID: 1304217 Review. German.
-
Drusen in age-related macular degeneration: pathogenesis, natural course, and laser photocoagulation-induced regression.Surv Ophthalmol. 1999 Jul-Aug;44(1):1-29. doi: 10.1016/s0039-6257(99)00072-7. Surv Ophthalmol. 1999. PMID: 10466585 Review.
Cited by
-
HISTOLOGY, DIMENSIONS, AND FLUORESCEIN STAINING CHARACTERISTICS OF NODULAR AND CUTICULAR DRUSEN IN AGE-RELATED MACULAR DEGENERATION.Retina. 2023 Oct 1;43(10):1708-1716. doi: 10.1097/IAE.0000000000003871. Retina. 2023. PMID: 37399252 Free PMC article.
-
Age-Related Scattered Hypofluorescent Spots as an Adverse Prognostic Factor for Polypoidal Choroidal Vasculopathy.Ophthalmol Sci. 2025 May 2;5(5):100818. doi: 10.1016/j.xops.2025.100818. eCollection 2025 Sep-Oct. Ophthalmol Sci. 2025. PMID: 40547001 Free PMC article.
-
Identification of Structures Labeled by Indocyanine Green in the Rat Choroid and Retina Can Guide Interpretation of Indocyanine Green Angiography.Invest Ophthalmol Vis Sci. 2024 Jan 2;65(1):25. doi: 10.1167/iovs.65.1.25. Invest Ophthalmol Vis Sci. 2024. PMID: 38193758 Free PMC article.
-
Structural integrity of retinal pigment epithelial cells in eyes with age-related scattered hypofluorescent spots on late phase indocyanine green angiography (ASHS-LIA).Eye (Lond). 2023 Feb;37(2):377-378. doi: 10.1038/s41433-022-02232-x. Epub 2022 Sep 17. Eye (Lond). 2023. PMID: 36115884 Free PMC article. No abstract available.
-
Pachychoroid disease: review and update.Eye (Lond). 2025 Apr;39(5):819-834. doi: 10.1038/s41433-024-03253-4. Epub 2024 Aug 3. Eye (Lond). 2025. PMID: 39095470 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

