Tether-free photothermal deep-brain stimulation in freely behaving mice via wide-field illumination in the near-infrared-II window
- PMID: 35314800
- PMCID: PMC9232843
- DOI: 10.1038/s41551-022-00862-w
Tether-free photothermal deep-brain stimulation in freely behaving mice via wide-field illumination in the near-infrared-II window
Abstract
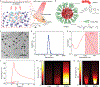
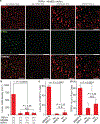
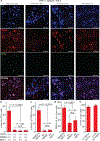
Neural circuitry is typically modulated via invasive brain implants and tethered optical fibres in restrained animals. Here we show that wide-field illumination in the second near-infrared spectral window (NIR-II) enables implant-and-tether-free deep-brain stimulation in freely behaving mice with stereotactically injected macromolecular photothermal transducers activating neurons ectopically expressing the temperature-sensitive transient receptor potential cation channel subfamily V member 1 (TRPV1). The macromolecular transducers, ~40 nm in size and consisting of a semiconducting polymer core and an amphiphilic polymer shell, have a photothermal conversion efficiency of 71% at 1,064 nm, the wavelength at which light attenuation by brain tissue is minimized (within the 400-1,800 nm spectral window). TRPV1-expressing neurons in the hippocampus, motor cortex and ventral tegmental area of mice can be activated with minimal thermal damage on wide-field NIR-II illumination from a light source placed at distances higher than 50 cm above the animal's head and at an incident power density of 10 mW mm-2. Deep-brain stimulation via wide-field NIR-II illumination may open up opportunities for social behavioural studies in small animals.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures



Similar articles
-
Protocol for wireless deep brain stimulation in freely behaving mice with infrared light.STAR Protoc. 2023 Mar 17;4(1):101757. doi: 10.1016/j.xpro.2022.101757. Epub 2022 Dec 19. STAR Protoc. 2023. PMID: 36538396 Free PMC article.
-
Antibody-conjugated gold nanoparticles as nanotransducers for second near-infrared photo-stimulation of neurons in rats.Nano Converg. 2022 Mar 21;9(1):13. doi: 10.1186/s40580-022-00304-y. Nano Converg. 2022. PMID: 35312875 Free PMC article.
-
Tetherless near-infrared control of brain activity in behaving animals using fully implantable upconversion microdevices.Biomaterials. 2017 Oct;142:136-148. doi: 10.1016/j.biomaterials.2017.07.017. Epub 2017 Jul 12. Biomaterials. 2017. PMID: 28735174
-
Nanotransducer-Enabled Deep-Brain Neuromodulation with NIR-II Light.ACS Nano. 2023 May 9;17(9):7941-7952. doi: 10.1021/acsnano.2c12068. Epub 2023 Apr 20. ACS Nano. 2023. PMID: 37079455 Review.
-
Recent Advances of NIR-II Emissive Semiconducting Polymer Dots for In Vivo Tumor Fluorescence Imaging and Theranostics.Biosensors (Basel). 2022 Dec 5;12(12):1126. doi: 10.3390/bios12121126. Biosensors (Basel). 2022. PMID: 36551093 Free PMC article. Review.
Cited by
-
Gold Nanorod-Embedded PDMS Micro-Pillar Array for Localized Photothermal Stimulation.J Microelectromech Syst. 2024 Oct;33(5):543-549. doi: 10.1109/jmems.2024.3418373. Epub 2024 Jul 3. J Microelectromech Syst. 2024. PMID: 39364062 Free PMC article.
-
Furan Donor for NIR-II Molecular Fluorophores with Enhanced Bioimaging Performance.Research (Wash D C). 2023;6:0039. doi: 10.34133/research.0039. Epub 2023 Jan 13. Research (Wash D C). 2023. PMID: 37040521 Free PMC article.
-
Mid-Infrared Photoacoustic Stimulation of Neurons through Vibrational Excitation in Polydimethylsiloxane.Adv Sci (Weinh). 2024 Sep;11(35):e2405677. doi: 10.1002/advs.202405677. Epub 2024 Jul 12. Adv Sci (Weinh). 2024. PMID: 38994890 Free PMC article.
-
Beyond 25 years of biomedical innovation in nano-bioelectronics.Device. 2024 Jul 19;2(7):100401. doi: 10.1016/j.device.2024.100401. Device. 2024. PMID: 39119268 Free PMC article.
-
Theoretical analysis of low-power deep synergistic sono-optogenetic excitation of neurons by co-expressing light-sensitive and mechano-sensitive ion-channels.Commun Biol. 2025 Mar 6;8(1):379. doi: 10.1038/s42003-025-07792-8. Commun Biol. 2025. PMID: 40050670 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

