Asymmetric peptidoglycan editing generates cell curvature in Bdellovibrio predatory bacteria
- PMID: 35314810
- PMCID: PMC8938487
- DOI: 10.1038/s41467-022-29007-y
Asymmetric peptidoglycan editing generates cell curvature in Bdellovibrio predatory bacteria
Abstract
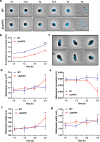
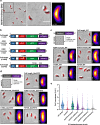
Peptidoglycan hydrolases contribute to the generation of helical cell shape in Campylobacter and Helicobacter bacteria, while cytoskeletal or periskeletal proteins determine the curved, vibrioid cell shape of Caulobacter and Vibrio. Here, we identify a peptidoglycan hydrolase in the vibrioid-shaped predatory bacterium Bdellovibrio bacteriovorus which invades and replicates within the periplasm of Gram-negative prey bacteria. The protein, Bd1075, generates cell curvature in B. bacteriovorus by exerting LD-carboxypeptidase activity upon the predator cell wall as it grows inside spherical prey. Bd1075 localizes to the outer convex face of B. bacteriovorus; this asymmetric localization requires a nuclear transport factor 2-like (NTF2) domain at the protein C-terminus. We solve the crystal structure of Bd1075, which is monomeric with key differences to other LD-carboxypeptidases. Rod-shaped Δbd1075 mutants invade prey more slowly than curved wild-type predators and stretch invaded prey from within. We therefore propose that the vibrioid shape of B. bacteriovorus contributes to predatory fitness.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Stolp H, Starr MP. Bdellovibrio bacteriovorus gen. et sp. n., a predatory, ectoparasitic, and bacteriolytic microorganism. Antonie Van. Leeuwenhoek. 1963;29:217–248. - PubMed
-
- Dashiff A, Junka RA, Libera M, Kadouri DE. Predation of human pathogens by the predatory bacteria Micavibrio aeruginosavorus and Bdellovibrio bacteriovorus. J. Appl. Microbiol. 2011;110:431–444. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

