DNA is loaded through the 9-1-1 DNA checkpoint clamp in the opposite direction of the PCNA clamp
- PMID: 35314830
- PMCID: PMC9010301
- DOI: 10.1038/s41594-022-00742-6
DNA is loaded through the 9-1-1 DNA checkpoint clamp in the opposite direction of the PCNA clamp
Abstract
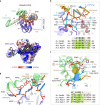
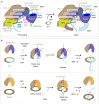
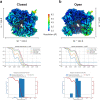
The 9-1-1 DNA checkpoint clamp is loaded onto 5'-recessed DNA to activate the DNA damage checkpoint that arrests the cell cycle. The 9-1-1 clamp is a heterotrimeric ring that is loaded in Saccharomyces cerevisiae by Rad24-RFC (hRAD17-RFC), an alternate clamp loader in which Rad24 replaces Rfc1 in the RFC1-5 clamp loader of proliferating cell nuclear antigen (PCNA). The 9-1-1 clamp loading mechanism has been a mystery, because, unlike RFC, which loads PCNA onto a 3'-recessed junction, Rad24-RFC loads the 9-1-1 ring onto a 5'-recessed DNA junction. Here we report two cryo-EM structures of Rad24-RFC-DNA with a closed or 27-Å open 9-1-1 clamp. The structures reveal a completely unexpected mechanism by which a clamp can be loaded onto DNA. Unlike RFC, which encircles DNA, Rad24 binds 5'-DNA on its surface, not inside the loader, and threads the 3' ssDNA overhang into the 9-1-1 clamp from above the ring.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








Similar articles
-
Unexpected new insights into DNA clamp loaders: Eukaryotic clamp loaders contain a second DNA site for recessed 5' ends that facilitates repair and signals DNA damage: Eukaryotic clamp loaders contain a second DNA site for recessed 5' ends that facilitates repair and signals DNA damage.Bioessays. 2022 Nov;44(11):e2200154. doi: 10.1002/bies.202200154. Epub 2022 Sep 18. Bioessays. 2022. PMID: 36116108 Free PMC article.
-
Replication factor C is a more effective proliferating cell nuclear antigen (PCNA) opener than the checkpoint clamp loader, Rad24-RFC.J Biol Chem. 2012 Jan 13;287(3):2203-9. doi: 10.1074/jbc.C111.318899. Epub 2011 Nov 24. J Biol Chem. 2012. PMID: 22115746 Free PMC article.
-
Structures of 9-1-1 DNA checkpoint clamp loading at gaps from start to finish and ramification on biology.Cell Rep. 2023 Jul 25;42(7):112694. doi: 10.1016/j.celrep.2023.112694. Epub 2023 Jun 30. Cell Rep. 2023. PMID: 37392384 Free PMC article.
-
Functions of Multiple Clamp and Clamp-Loader Complexes in Eukaryotic DNA Replication.Adv Exp Med Biol. 2017;1042:135-162. doi: 10.1007/978-981-10-6955-0_7. Adv Exp Med Biol. 2017. PMID: 29357057 Review.
-
The PCNA-RFC families of DNA clamps and clamp loaders.Prog Nucleic Acid Res Mol Biol. 2004;78:227-60. doi: 10.1016/S0079-6603(04)78006-X. Prog Nucleic Acid Res Mol Biol. 2004. PMID: 15210332 Review.
Cited by
-
Resection of DNA double-strand breaks activates Mre11-Rad50-Nbs1- and Rad9-Hus1-Rad1-dependent mechanisms that redundantly promote ATR checkpoint activation and end processing in Xenopus egg extracts.Nucleic Acids Res. 2024 Apr 12;52(6):3146-3163. doi: 10.1093/nar/gkae082. Nucleic Acids Res. 2024. PMID: 38349040 Free PMC article.
-
The human ATAD5 has evolved unique structural elements to function exclusively as a PCNA unloader.Nat Struct Mol Biol. 2024 Nov;31(11):1680-1691. doi: 10.1038/s41594-024-01332-4. Epub 2024 Jun 13. Nat Struct Mol Biol. 2024. PMID: 38871854 Free PMC article.
-
Unexpected new insights into DNA clamp loaders: Eukaryotic clamp loaders contain a second DNA site for recessed 5' ends that facilitates repair and signals DNA damage: Eukaryotic clamp loaders contain a second DNA site for recessed 5' ends that facilitates repair and signals DNA damage.Bioessays. 2022 Nov;44(11):e2200154. doi: 10.1002/bies.202200154. Epub 2022 Sep 18. Bioessays. 2022. PMID: 36116108 Free PMC article.
-
PCNA is a Nucleotide Exchange Factor for the Clamp Loader ATPase Complex.bioRxiv [Preprint]. 2025 Jul 3:2025.07.02.662830. doi: 10.1101/2025.07.02.662830. bioRxiv. 2025. PMID: 40631102 Free PMC article. Preprint.
-
The Atad5 RFC-like complex is the major unloader of proliferating cell nuclear antigen in Xenopus egg extracts.J Biol Chem. 2024 Jan;300(1):105588. doi: 10.1016/j.jbc.2023.105588. Epub 2023 Dec 21. J Biol Chem. 2024. PMID: 38141767 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

