Eicosanoid signalling blockade protects middle-aged mice from severe COVID-19
- PMID: 35314834
- PMCID: PMC9783543
- DOI: 10.1038/s41586-022-04630-3
Eicosanoid signalling blockade protects middle-aged mice from severe COVID-19
Abstract
Coronavirus disease 2019 (COVID-19) is especially severe in aged populations1. Vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are highly effective, but vaccine efficacy is partly compromised by the emergence of SARS-CoV-2 variants with enhanced transmissibility2. The emergence of these variants emphasizes the need for further development of anti-SARS-CoV-2 therapies, especially for aged populations. Here we describe the isolation of highly virulent mouse-adapted viruses and use them to test a new therapeutic drug in infected aged animals. Many of the alterations observed in SARS-CoV-2 during mouse adaptation (positions 417, 484, 493, 498 and 501 of the spike protein) also arise in humans in variants of concern2. Their appearance during mouse adaptation indicates that immune pressure is not required for selection. For murine SARS, for which severity is also age dependent, elevated levels of an eicosanoid (prostaglandin D2 (PGD2)) and a phospholipase (phospholipase A2 group 2D (PLA2G2D)) contributed to poor outcomes in aged mice3,4. mRNA expression of PLA2G2D and prostaglandin D2 receptor (PTGDR), and production of PGD2 also increase with ageing and after SARS-CoV-2 infection in dendritic cells derived from human peripheral blood mononuclear cells. Using our mouse-adapted SARS-CoV-2, we show that middle-aged mice lacking expression of PTGDR or PLA2G2D are protected from severe disease. Furthermore, treatment with a PTGDR antagonist, asapiprant, protected aged mice from lethal infection. PTGDR antagonism is one of the first interventions in SARS-CoV-2-infected animals that specifically protects aged animals, suggesting that the PLA2G2D-PGD2/PTGDR pathway is a useful target for therapeutic interventions.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Figures

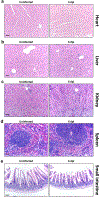







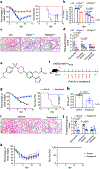

References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

