A Comprehensive Investigation Regarding the Differentiation of the Procurable COVID-19 Vaccines
- PMID: 35314902
- PMCID: PMC8936379
- DOI: 10.1208/s12249-022-02247-3
A Comprehensive Investigation Regarding the Differentiation of the Procurable COVID-19 Vaccines
Abstract
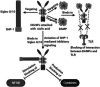
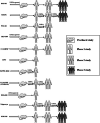
COVID-19 caused by coronavirus SARS-CoV-2 became a serious threat to humankind for the past couple of years. The development of vaccine and its immediate application might be the only to escape from the grasp of this demoniac pandemic. Approximately 343 clinical trials on COVID-19 vaccines are ongoing currently, and almost all countries are motivating ongoing researches at warp speed for the development of vaccines against COVID-19. This review explores the progress in the development of the vaccines, their current status of ongoing clinical research, mechanisms, and regulatory approvals. Many pharmaceutical companies are already in the endgame for manufacturing various vaccines of which some are already being marketed across the globe, while others are yet to get approval for marketing. The primary aim of this review is to compare regulatory accepted vaccines in terms of their composition, doses, regulatory status, and efficacy. The study is conducted by grouping into approved and unapproved vaccines for marketing. Different routes of administration of vaccines along with the efficacy of the routes are also presented in the review. A wide range of database and clinical trial data is reviewed for sorting out the information on different vaccines. Unfortunately, many mutations (alpha, beta, gamma, delta, kappa, omicron etc.) of SARS-CoV-2 have attacked people in very short time, which is the great challenge for investigational vaccines. Moreover, some vaccines like Pfizer's BNT162, Oxford's ChAdOx1, Moderna's mRNA-1273, and Bharat Biotech's Covaxin have got regulatory approval in some countries for its distribution which may prove to stand tall against the pandemic.
Keywords: clinical trials; covid-19; prevention; sars-cov-2; vaccines.
© 2022. The Author(s), under exclusive licence to American Association of Pharmaceutical Scientists.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Inactivated vaccine Covaxin/BBV152: A systematic review.Front Immunol. 2022 Aug 9;13:863162. doi: 10.3389/fimmu.2022.863162. eCollection 2022. Front Immunol. 2022. PMID: 36016940 Free PMC article.
-
The Rapid Development and Early Success of Covid 19 Vaccines Have Raised Hopes for Accelerating the Cancer Treatment Mechanism.Arch Razi Inst. 2021 Mar;76(1):1-6. doi: 10.22092/ari.2021.353761.1612. Epub 2021 Mar 1. Arch Razi Inst. 2021. PMID: 33818952 Free PMC article.
-
Novel corona virus (COVID-19); Global efforts and effective investigational medicines: A review.J Infect Public Health. 2021 Jul;14(7):910-921. doi: 10.1016/j.jiph.2021.04.011. Epub 2021 May 1. J Infect Public Health. 2021. PMID: 34119845 Free PMC article. Review.
-
Disease severity and efficacy of homologous vaccination among patients infected with SARS-CoV-2 Delta or Omicron VOCs, compared to unvaccinated using main biomarkers.J Med Virol. 2022 Dec;94(12):5867-5876. doi: 10.1002/jmv.28098. Epub 2022 Sep 9. J Med Virol. 2022. PMID: 36029103 Free PMC article.
-
COVID-19: morphology and mechanism of the SARS-CoV-2, global outbreak, medication, vaccines and future of the virus.Front Biosci (Elite Ed). 2021 Dec 20;13(2):272-290. doi: 10.52586/E884. Front Biosci (Elite Ed). 2021. PMID: 34937314 Review.
Cited by
-
Optimized lipid nanoparticles (LNPs) for organ-selective nucleic acids delivery in vivo.iScience. 2024 Apr 23;27(6):109804. doi: 10.1016/j.isci.2024.109804. eCollection 2024 Jun 21. iScience. 2024. PMID: 38770138 Free PMC article. Review.
-
A Clinical Insight on New Discovered Molecules and Repurposed Drugs for the Treatment of COVID-19.Vaccines (Basel). 2023 Feb 1;11(2):332. doi: 10.3390/vaccines11020332. Vaccines (Basel). 2023. PMID: 36851211 Free PMC article. Review.
-
Incorporation of a Toll-like receptor 2/6 agonist potentiates mRNA vaccines against cancer and infectious diseases.Signal Transduct Target Ther. 2023 Jul 17;8(1):273. doi: 10.1038/s41392-023-01479-4. Signal Transduct Target Ther. 2023. PMID: 37455272 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

