Being Transparent About Brilliant Failures: An Attempt to Use Real-World Data in a Disease Model for Patients with Castration-Resistant Prostate Cancer
- PMID: 35314962
- PMCID: PMC9114194
- DOI: 10.1007/s40801-022-00294-7
Being Transparent About Brilliant Failures: An Attempt to Use Real-World Data in a Disease Model for Patients with Castration-Resistant Prostate Cancer
Abstract
Background: Real-world disease models spanning multiple treatment lines can provide insight into the (cost) effectiveness of treatment sequences in clinical practice.
Objective: Our objective was to explore whether a disease model based solely on real-world data (RWD) could be used to estimate the effectiveness of treatments for patients with castration-resistant prostate cancer (CRPC) that could then be suitably used in a cost-effectiveness analysis.
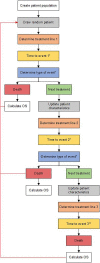
Methods: We developed a patient-level simulation model using patient-level data from the Dutch CAPRI registry as input parameters. Time to event (TTE) and overall survival (OS) were estimated with multivariate regression models, and type of event (i.e., next treatment or death) was estimated with multivariate logistic regression models. To test internal validity, TTE and OS from the simulation model were compared with the observed outcomes in the registry.
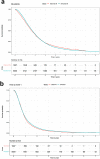
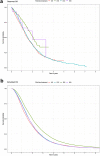
Results: Although patient characteristics and survival outcomes of the simulated data were comparable to those in the observed data (median OS 20.6 vs. 19.8 months, respectively), the disease model was less accurate in estimating differences between treatments (median OS simulated vs. observed population: 18.6 vs. 17.9 [abiraterone acetate plus prednisone], 24.0 vs. 25.0 [enzalutamide], 20.2 vs. 18.7 [docetaxel], and 20.0 vs. 23.8 months [radium-223]).
Conclusions: Overall, the disease model accurately approximated the observed data in the total CRPC population. However, the disease model was unable to predict differences in survival between treatments due to unobserved differences. Therefore, the model is not suitable for cost-effectiveness analysis of CRPC treatment. Using a combination of RWD and data from randomised controlled trials to estimate treatment effectiveness may improve the model.
© 2022. The Author(s).
Conflict of interest statement
Marscha S. Holleman, Simone A. Huygens, Maiwenn J. Al, Malou C.P. Kuppen, Hans M. Westgeest, Alfonsus C.M. van den Bergh, Andries M. Bergman, Alfonsus J.M. van den Eertwegh, Mathijs P. Hendriks, Menuhin I. Lampe, Niven Mehra, Reindert J.A. van Moorselaar, Inge M. van Oort, Diederik M. Somford, Ronald de Wit, Agnes J. van de Wouw, Winald R. Gerritsen, and Carin A. Uyl-de Groot have no conflicts of interest that are directly relevant to the content of this article.
Figures
References
-
- Nederlandse Kankerregistratie. In: No title. 2019. https://www.cijfersoverkanker.nl/. Accessed Feb 2019.
-
- Cornford P, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, Fanti S, Fossati N, Gandaglia G, Gillessen S, Grivas N, Grummet J, Henry AM, der Kwast THV, Lam TB, Lardas M, Liew M, Mason MD, Moris L, Oprea-Lager DE, der Poel HGV, Rouvière O, Schoots IG, Tilki D, Wiegel T, Willemse PM, Mottet N. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. 2021;79:263–282. doi: 10.1016/j.eururo.2020.09.046. - DOI - PubMed
-
- Nederlandse Vereniging voor Urologie. In: Prostaatcarcinoom. 2019. https://www.oncoline.nl/index.php?pagina=/richtlijn/item/pagina.php&rich.... 2020.
-
- Parker CC, James ND, Brawley CD, Clarke NW, Hoyle AP, Ali A, Ritchie AWS, Attard G, Chowdhury S, Cross W, Dearnaley DP, Gillessen S, Gilson C, Jones RJ, Langley RE, Malik ZI, Mason MD, Matheson D, Millman R, Russell JM, Thalmann GN, Amos CL, Alonzi R, Bahl A, Birtle A, Din O, Douis H, Eswar C, Gale J, Gannon MR, Jonnada S, Khaksar S, Lester JF, O'Sullivan J, Parikh OA, Pedley ID, Pudney DM, Sheehan DJ, Srihari NN, Tran ATH, Parmar MKB, Sydes MR. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial Invalid date. Lancet. 2018;392:2353–2366. doi: 10.1016/S0140-6736(18)32486-3. - DOI - PMC - PubMed
-
- Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, Mottet N. EAU Guidelines on Prostate Cancer. Part II: Treatment of Advanced, Relapsing, and Castration-Resistant Prostate CancerInvalid date. Eur Urol. 2013 doi: 10.1016/j.eururo.2013.11.002. - DOI - PubMed
LinkOut - more resources
Full Text Sources

