THBS2 promotes cell migration and invasion in colorectal cancer via modulating Wnt/β-catenin signaling pathway
- PMID: 35315209
- PMCID: PMC11896594
- DOI: 10.1002/kjm2.12528
THBS2 promotes cell migration and invasion in colorectal cancer via modulating Wnt/β-catenin signaling pathway
Abstract
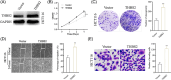
Colorectal cancer (CRC) is the most common human digestive malignancy with a poor prognosis; the pathophysiology of colon cancer involves multiple linkages of regulatory networks. Recently, thrombospondin 2 (THBS2) has been extensively studied for its role in cancer progression. In this study, we evaluated the expression of THBS2 in CRC tissues and studied the possible mechanism by which THBS2 regulates CRC progression. Our results showed that the upregulation of THBS2 in CRC tissues and CRC cell lines and high expression of THBS2 was correlated with poor overall survival. The in vitro experimental data showed that THBS2 overexpression promoted CRC cell growth, invasion, and migration, while THBS2 inhibition exerted tumor-suppressive actions on CRC cells. THBS2 knockdown suppressed the activity of Wnt/β-catenin signaling. Collectively, the results implied that THBS2 exerted promotional effects on CRC cell proliferation, invasion, and migration, partly by modulating the Wnt/β-catenin signaling pathway.
Keywords: THBS2; Wnt/β-catenin; colon cancer; invasion; migration.
© 2022 The Authors. The Kaohsiung Journal of Medical Sciences published by John Wiley & Sons Australia, Ltd on behalf of Kaohsiung Medical University.
Conflict of interest statement
All authors declare no conflict of interest.
Figures




References
-
- Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145–64. - PubMed
-
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–64. - PubMed
-
- Liu CC, Cai DL, Sun F, Wu ZH, Yue B, Zhao SL, et al. FERMT1 mediates epithelial‐mesenchymal transition to promote colon cancer metastasis via modulation of β‐catenin transcriptional activity. Oncogene. 2017;36(13):1779–92. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

