Tocilizumab in addition to standard of care in the management of COVID-19: a meta-analysis of RCTs
- PMID: 35315395
- PMCID: PMC8972884
- DOI: 10.23750/abm.v93i1.12208
Tocilizumab in addition to standard of care in the management of COVID-19: a meta-analysis of RCTs
Abstract
Objective: We performed a systematic review and meta-analysis for exploring clinical benefits and safety of tocilizumab in addition to standard of care (SOC) in treating patients with coronavirus disease 2019 (COVID-19).
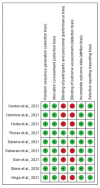
Methods: An electronic search was carried out in PubMed, EMBASE, Cochrane Library, and Science Direct, as well as in medRxiv preprint server, to identify eligible studies. Only randomized Controlled Trials (RCTs) that compared mortality events and/or adverse events between a tocilizumab + SOC group and a SOC-only control group were included. The primary outcome was 28-day mortality. Secondary outcomes include progression to severe disease, defined as need for mechanical ventilation (MV) or intensive care unit (ICU) admission, and adverse events (AE).
Results: A total of nine studies (6,490 participants) could be included in this meta-analysis, with 3,358 participants in the tocilizumab + SOC group and 3,132 participants in the SOC-only group. The overall mortality rate was lower in the tocilizumab group compared to the SOC-only group, though the difference was not statistically significant (odds ratio [OR], 0.87; 95% CI, 0.73-1.04; I2, 15%). This finding was unaffected by subgroup analyses based on initial use of steroids or mechanical ventilation at baseline. Patients receiving tocilizumab were 26% less likely to progress to MV, and this difference was statistically significant (OR, 0.74; 95% CI, 0.64-0.86; I2, 0%). Among patients who were not in ICU at randomization, the tocilizumab group had 34 % lower rate of ICU admission compared to the SOC-only group (OR, 0.66; 95% CI, 0.40-2.14; I2, 29%). The occurrence of serious infections was lower in the tocilizumab group (OR, 0.57; 95% CI, 0.36-0.89; I2, 21%).
Conclusion: Tocilizumab is generally well-tolerated in COVID-19. Although this drug does not appear to have a significant benefits on survival, it may have a role in preventing progression to intensive care and MV.
Conflict of interest statement
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
Figures










References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

