Endothelial pannexin-1 channels modulate macrophage and smooth muscle cell activation in abdominal aortic aneurysm formation
- PMID: 35315432
- PMCID: PMC8938517
- DOI: 10.1038/s41467-022-29233-4
Endothelial pannexin-1 channels modulate macrophage and smooth muscle cell activation in abdominal aortic aneurysm formation
Abstract
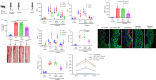
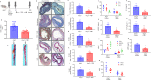
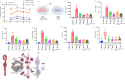
Pannexin-1 (Panx1) channels have been shown to regulate leukocyte trafficking and tissue inflammation but the mechanism of Panx1 in chronic vascular diseases like abdominal aortic aneurysms (AAA) is unknown. Here we demonstrate that Panx1 on endothelial cells, but not smooth muscle cells, orchestrate a cascade of signaling events to mediate vascular inflammation and remodeling. Mechanistically, Panx1 on endothelial cells acts as a conduit for ATP release that stimulates macrophage activation via P2X7 receptors and mitochondrial DNA release to increase IL-1β and HMGB1 secretion. Secondly, Panx1 signaling regulates smooth muscle cell-dependent intracellular Ca2+ release and vascular remodeling via P2Y2 receptors. Panx1 blockade using probenecid markedly inhibits leukocyte transmigration, aortic inflammation and remodeling to mitigate AAA formation. Panx1 expression is upregulated in human AAAs and retrospective clinical data demonstrated reduced mortality in aortic aneurysm patients treated with Panx1 inhibitors. Collectively, these data identify Panx1 signaling as a contributory mechanism of AAA formation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat. Rev. Cardiol. 2011;8:92–102. - PubMed
-
- Sakalihasan N, et al. Abdominal aortic aneurysms. Nat. Rev. Dis. Prim. 2018;4:34. - PubMed
-
- Harris LM, Faggioli GL, Fiedler R, Curl GR, Ricotta JJ. Ruptured abdominal aortic aneurysms: factors affecting mortality rates. J. Vasc. Surg. 1991;14:812–818. - PubMed
-
- Golledge J. Abdominal aortic aneurysm: update on pathogenesis and medical treatments. Nat. Rev. Cardiol. 2019;16:225–242. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

