Feature tracking microfluidic analysis reveals differential roles of viscosity and friction in sickle cell blood
- PMID: 35315465
- PMCID: PMC9004467
- DOI: 10.1039/d1lc01133b
Feature tracking microfluidic analysis reveals differential roles of viscosity and friction in sickle cell blood
Abstract
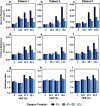
Characterization of blood flow rheology in hematological disorders is critical for understanding disease pathophysiology. Existing methods to measure blood rheological parameters are limited in their physiological relevance, and there is a need for new tools that focus on the microcirculation and extract properties at finer resolution than overall flow resistance. Herein, we present a method that combines microfluidic systems and powerful object-tracking computational technologies with mathematical modeling to separate the red blood cell flow profile into a bulk component and a wall component. We use this framework to evaluate differential contributions of effective viscosity and wall friction to the overall resistance in blood from patients with sickle cell disease (SCD) under a range of oxygen tensions. Our results demonstrate that blood from patients with SCD exhibits elevated frictional and viscous resistances at all physiologic oxygen tensions. Additionally, the viscous resistance increases more rapidly than the frictional resistance as oxygen tension decreases, which may confound analyses that extract only flow velocities or overall flow resistances. Furthermore, we evaluate the impact of transfusion treatments on the components of the resistance, revealing patient variability in blood properties that may improve our understanding of the heterogeneity of clinical responses to such treatments. Overall, our system provides a new method to analyze patient-specific blood properties and can be applied to a wide range of hematological and vascular disorders.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

