Feasibility evaluation of the transapical saddle-shaped valved stent for transcatheter mitral valve implantation
- PMID: 35315544
- PMCID: PMC9315026
- DOI: 10.1111/jocs.16426
Feasibility evaluation of the transapical saddle-shaped valved stent for transcatheter mitral valve implantation
Abstract
Background and aims of study: Transcatheter mitral valve implantation (TMVI) is a promising and minimally invasive treatment for high-risk mitral regurgitation. We aimed to investigate the feasibility of a novel self-expanding valved stent for TMVI via apical access.
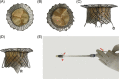
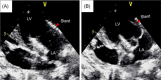
Methods: We designed a novel self-expanding mitral valve stent system consisting of an atrial flange and saddle-shaped ventricular body connected by two opposing anchors and two opposing extensions. During valve deployment, each anchor was controlled by a recurrent string. TMVI was performed in 10 pigs using the valve prosthesis through apical access to verify technical feasibility. Echocardiography and ventricular angiography were used to assess hemodynamic data and valve function. Surviving pigs were killed 4 weeks later to confirm stent deployment.
Results: Ten animals underwent TMVI using the novel mitral valve stent. Optimal valve deployment and accurate anatomical adjustments were obtained in nine animals. Implantation failed in one case, and the animal died 1 day later due to stent mismatch. After stent implantation, the hemodynamic parameters of the other animals were stable, and valve function was normal. The mean pressure across the mitral valve and left ventricular outflow tract were 2.98 ± 0.91 mmHg and 3.42 ± 0.66 mmHg, respectively. Macroscopic evaluation confirmed the stable and secure positioning of the stents. No obvious valve displacement, embolism, or paravalvular leakage was observed 4 weeks postvalve implantation.
Conclusions: This study demonstrated that the novel mitral valve is technically feasible in animals. However, the long-term feasibility and durability of this valved stent must be improved and verified.
Keywords: mitral regurgitation; transcatheter mitral valve implantation; valve prosthesis.
© 2022 The Authors. Journal of Cardiac Surgery published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
New stent for transapical mitral valve replacement in acute swine experiment.J Cardiothorac Surg. 2021 Apr 21;16(1):101. doi: 10.1186/s13019-021-01483-1. J Cardiothorac Surg. 2021. PMID: 33882974 Free PMC article.
-
Mitral valved stent implantation.Eur J Cardiothorac Surg. 2010 Sep;38(3):350-5. doi: 10.1016/j.ejcts.2010.02.022. Epub 2010 Mar 26. Eur J Cardiothorac Surg. 2010. PMID: 20347323
-
Off-pump transapical mitral valve replacement.Eur J Cardiothorac Surg. 2009 Jul;36(1):124-8; discussion 128. doi: 10.1016/j.ejcts.2009.02.037. Epub 2009 Apr 25. Eur J Cardiothorac Surg. 2009. PMID: 19394858
-
[Interventional mitral valve replacement. Current status].Herz. 2016 Feb;41(1):31-6. doi: 10.1007/s00059-015-4389-x. Herz. 2016. PMID: 26660091 Review. German.
-
Transcatheter Mitral Valve Replacement for Native and Failed Bioprosthetic Mitral Valves.Methodist Debakey Cardiovasc J. 2017 Jul-Sep;13(3):142-151. doi: 10.14797/mdcj-13-3-142. Methodist Debakey Cardiovasc J. 2017. PMID: 29743999 Free PMC article. Review.
References
-
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez‐Sarano M. Burden of valvular heart diseases: a population‐based study. Lancet. 2006;368(9540):1005‐1011. - PubMed
-
- Mirabel M, Iung B, Baron G, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J. 2007;28(11):1358‐1365. - PubMed
-
- Iung B, Vahanian A. Epidemiology of valvular heart disease in the adult. Nat Rev Cardiol. 2011;8(3):162‐172. - PubMed
-
- Patel A, Bapat V. Transcatheter mitral valve replacement: device landscape and early results. EuroIntervention. 2017;13:AA31‐AA39. - PubMed
-
- Duncan A. Transcatheter mitral valve replacement: the Tendyne device. Transcatheter Mitral Valve Therapies, Wiley Online Library; 2021:261‐275.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

