Growth Responses During 3 Years of Growth Hormone Treatment in Children and Adolescents With Growth Hormone Deficiency: Comparison Between Idiopathic, Organic and Isolated Growth Hormone Deficiency, and Multiple Pituitary Hormone Deficiency
- PMID: 35315601
- PMCID: PMC8938607
- DOI: 10.3346/jkms.2022.37.e90
Growth Responses During 3 Years of Growth Hormone Treatment in Children and Adolescents With Growth Hormone Deficiency: Comparison Between Idiopathic, Organic and Isolated Growth Hormone Deficiency, and Multiple Pituitary Hormone Deficiency
Abstract
Background: The study aimed to compare the growth responses to 3 years of growth hormone (GH) treatment in children and adolescents with GH deficiency (GHD) according to idiopathic, organic, isolated (IGHD), and multiple pituitary hormone deficiency (MPHD).
Methods: Total 163 patients aged 2-18 years (100 males and 63 females; 131 idiopathic and 32 organic GHD; 129 IGHD and 34 MPHD) were included from data obtained from the LG Growth Study. Parameters of growth responses and biochemical results were compared during the 3-year GH treatment.
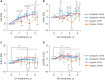
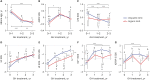
Results: The baseline age, bone age (BA), height (Ht) standard deviation score (SDS), weight SDS, mid-parental Ht SDS, predicted adult Ht (PAH) SDS, and insulin like growth factor-1 (IGF-1) SDS were significantly higher in the organic GHD patients than in the idiopathic GHD patients, but peak GH on the GH-stimulation test, baseline GH dose, and mean 3-year-GH dosage were higher in the idiopathic GHD patients than in the organic GHD patients. The prevalence of MPHD was higher in the organic GHD patients than in the idiopathic GHD patients. Idiopathic MPHD subgroup showed the largest increase for the ΔHt SDS and ΔPAH SDS during GH treatment, and organic MPHD subgroup had the smallest mean increase after GH treatment, depending on ΔIGF-1 SDS and ΔIGF binding protein-3 (IGFBP-3) SDS. The growth velocity and the parental-adjusted Ht gain were greater in the idiopathic GHD patients than the organic GHD patients during the 3-year GH treatment, which may have been related to the different GH dose, ΔIGF-1 SDS, and ΔIGFBP-3 SDS between two groups. Multiple linear regression analysis revealed that baseline IGF-1 SDS, BA, and MPH SDS in idiopathic group and baseline HT SDS in organic group are the most predictable parameters for favorable 3-year-GH treatment.
Conclusion: The 3-year-GH treatment was effective in both idiopathic and organic GHD patients regardless of the presence of MPHD or underlying causes, but their growth outcomes were not constant with each other. Close monitoring along with appropriate dosage of GH and annual growth responses, not specific at baseline, are more important in children and adolescents with GHD for long-term treatment.
Trial registration: ClinicalTrials.gov Identifier: NCT01604395.
Keywords: Adolescent; Child; Combined Pituitary Hormone Deficiency; Growth Hormone; Growth Hormone Deficiency.
© 2022 The Korean Academy of Medical Sciences.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures
Similar articles
-
Effect of growth hormone therapy on Taiwanese children with growth hormone deficiency.J Formos Med Assoc. 2012 Jul;111(7):355-63. doi: 10.1016/j.jfma.2011.06.011. Epub 2012 May 23. J Formos Med Assoc. 2012. PMID: 22817812
-
Response to growth hormone treatment in isolated growth hormone deficiency versus multiple pituitary hormone deficiency.Horm Res Paediatr. 2011;76 Suppl 1:42-6. doi: 10.1159/000329161. Epub 2011 Jul 21. Horm Res Paediatr. 2011. PMID: 21778748
-
Adult height in patients with permanent growth hormone deficiency with and without multiple pituitary hormone deficiencies.J Clin Endocrinol Metab. 2006 Aug;91(8):2900-5. doi: 10.1210/jc.2006-0050. Epub 2006 May 9. J Clin Endocrinol Metab. 2006. PMID: 16684828
-
Magnetic resonance images of 91 children with different causes of short stature: pituitary size reflects growth hormone secretion.Eur J Pediatr. 1997 Oct;156(10):758-63. doi: 10.1007/s004310050707. Eur J Pediatr. 1997. PMID: 9365063 Review.
-
The growth hormone cascade: progress and long-term results of growth hormone treatment in growth hormone deficiency.Horm Res. 1998;49 Suppl 2:41-57. doi: 10.1159/000053087. Horm Res. 1998. PMID: 9730672 Review.
Cited by
-
Impact of COVID-19 on growth hormone therapy efficacy in pediatric patients with short stature.Endocr Connect. 2025 Aug 7;14(8):e250218. doi: 10.1530/EC-25-0218. Print 2025 Aug 1. Endocr Connect. 2025. PMID: 40709714 Free PMC article.
-
Development and validation of a nomogram to predict poor short-term response to recombinant human growth hormone treatment in children with growth disorders.J Endocrinol Invest. 2023 Jul;46(7):1343-1359. doi: 10.1007/s40618-022-01979-0. Epub 2022 Dec 8. J Endocrinol Invest. 2023. PMID: 36480094
-
Comparative Efficacy of Once-Weekly Somatrogon Versus Daily Growth Hormone Therapy in Children With Idiopathic Growth Hormone Deficiency: A Real-World Retrospective Study From Greece.Cureus. 2025 Apr 25;17(4):e82998. doi: 10.7759/cureus.82998. eCollection 2025 Apr. Cureus. 2025. PMID: 40416193 Free PMC article.
-
Disrupted small-world networks in children with drug-naïve growth hormone deficiency: a DTI-based network analysis.Quant Imaging Med Surg. 2025 May 1;15(5):4101-4112. doi: 10.21037/qims-24-1927. Epub 2025 Apr 10. Quant Imaging Med Surg. 2025. PMID: 40384693 Free PMC article.
-
Construction and evaluation of a height prediction model for children with growth disorders treated with recombinant human growth hormone.BMC Endocr Disord. 2025 Jul 9;25(1):170. doi: 10.1186/s12902-025-01991-4. BMC Endocr Disord. 2025. PMID: 40634928 Free PMC article.
References
-
- Lindsay R, Feldkamp M, Harris D, Robertson J, Rallison M. Utah Growth Study: growth standards and the prevalence of growth hormone deficiency. J Pediatr. 1994;125(1):29–35. - PubMed
-
- Boguszewski MCS. Growth hormone deficiency and replacement in children. Rev Endocr Metab Disord. 2021;22(1):101–108. - PubMed
-
- Pozzobon G, Partenope C, Mora S, Garbetta G, Weber G, Barera G. Growth hormone therapy in children: predictive factors and short-term and long-term response criteria. Endocrine. 2019;66(3):614–621. - PubMed
-
- Kriström B, Aronson AS, Dahlgren J, Gustafsson J, Halldin M, Ivarsson SA, et al. Growth hormone (GH) dosing during catch-up growth guided by individual responsiveness decreases growth response variability in prepubertal children with GH deficiency or idiopathic short stature. J Clin Endocrinol Metab. 2009;94(2):483–490. - PubMed
-
- Bakker B, Frane J, Anhalt H, Lippe B, Rosenfeld RG. Height velocity targets from the national cooperative growth study for first-year growth hormone responses in short children. J Clin Endocrinol Metab. 2008;93(2):352–357. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

