Mycobacterium tuberculosis EspK Has Active but Distinct Roles in the Secretion of EsxA and EspB
- PMID: 35315684
- PMCID: PMC9017330
- DOI: 10.1128/jb.00060-22
Mycobacterium tuberculosis EspK Has Active but Distinct Roles in the Secretion of EsxA and EspB
Abstract
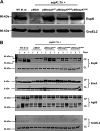
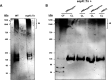
The Mycobacterium tuberculosis type-7 protein secretion system ESX-1 is a major driver of its virulence. While the functions of most ESX-1 components are characterized, many others remain poorly defined. In this study, we examined the role of EspK, an ESX-1-associated protein that is thought to be dispensable for ESX-1 activity in members of the Mycobacterium tuberculosis complex. We show that EspK is needed for the timely and optimal secretion of EsxA and absolutely essential for EspB secretion in M. tuberculosis Erdman. We demonstrate that only the EsxA secretion defect can be alleviated in EspK-deficient M. tuberculosis by culturing it in media containing detergents like Tween 80 or tyloxapol. Subcellular fractionation experiments reveal EspK is exported by M. tuberculosis in an ESX-1-independent manner and localized to its cell wall. We also show a conserved W-X-G motif in EspK is important for its interaction with EspB and enabling its secretion. The same motif, however, is not important for EspK localization in the cell wall. Finally, we show EspB in EspK-deficient M. tuberculosis tends to adopt higher-order oligomeric conformations, more so than EspB in wild-type M. tuberculosis. These results suggest EspK interacts with EspB and prevents it from assembling prematurely into macromolecular complexes that are presumably too large to pass through the membrane-spanning ESX-1 translocon assembly. Collectively, our findings indicate M. tuberculosis EspK has a far more active role in ESX-1-mediated secretion than was previously appreciated and underscores the complex nature of this secretion apparatus. IMPORTANCE Mycobacterium tuberculosis uses its ESX-1 system to secrete EsxA and EspB into a host to cause disease. We show that EspK, a protein whose role in the ESX-1 machinery was thought to be nonessential, is needed by M. tuberculosis for optimal EsxA and EspB secretion. Culturing EspK-deficient M. tuberculosis with detergents alleviates EsxA but not EspB secretion defects. We also show that EspK, which is exported by M. tuberculosis in an ESX-1-independent manner to the cell wall, interacts with and prevents EspB from assembling into large structures inside the M. tuberculosis cell that are nonsecretable. Collectively, our observations demonstrate EspK is an active component of the ESX-1 secretion machinery of the tubercle bacillus.
Keywords: ESX-1; EspK; Mycobacterium tuberculosis; protein secretion; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
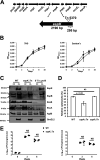
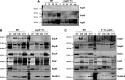
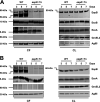
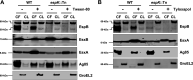


References
-
- Beckham KS, Ciccarelli L, Bunduc CM, Mertens HD, Ummels R, Lugmayr W, Mayr J, Rettel M, Savitski MM, Svergun DI, Bitter W, Wilmanns M, Marlovits TC, Parret AH, Houben EN. 2017. Structure of the mycobacterial ESX-5 type VII secretion system membrane complex by single-particle analysis. Nat Microbiol 2:17047. doi:10.1038/nmicrobiol.2017.47. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 31003A-140778/SNSF_/Swiss National Science Foundation/Switzerland
- 201762/European Community Seventh Framework Program (FP7)
- 260872/European Community Seventh Framework Program
- 05730/Gouvernement du Canada | Natural Sciences and Engineering Research Council of Canada (NSERC)
- National Sanitarium Association (NSA)
LinkOut - more resources
Full Text Sources
Medical

