Systemic Homologous Neutralizing Antibodies Are Inadequate for the Evaluation of Vaccine Protective Efficacy against Coinfection by High Virulent PEDV and PRRSV
- PMID: 35315711
- PMCID: PMC9045284
- DOI: 10.1128/spectrum.02574-21
Systemic Homologous Neutralizing Antibodies Are Inadequate for the Evaluation of Vaccine Protective Efficacy against Coinfection by High Virulent PEDV and PRRSV
Abstract
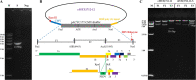
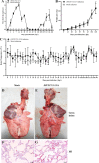
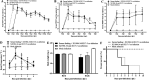
G2 porcine epidemic diarrhea virus (G2 PEDV) and highly pathogenic porcine reproductive and respiratory syndrome virus 2 (HP-PRRSV2) are two of the most prevalent swine pathogens in China's swine herds, and their coinfection occurs commonly. Several PED and PRRS vaccines have been utilized in China for decades, and systemic homologous neutralizing antibodies (shnAbs) in serum are frequently used to evaluate the protective efficacy of PED and PRRS vaccines. To develop a vaccine candidate against G2 PEDV and HP-PRRSV2 coinfection, in this study, we generated a chimeric virus (rJSTZ1712-12-S) expressing S protein of G2 PEDV using an avirulent HP-PRRSV2 rJSTZ1712-12 infectious clone as the viral vector. The rJSTZ1712-12-S strain has similar replication efficacies as the parental rJSTZ1712-12 virus. In addition, animal inoculation indicated that rJSTZ1712-12-S is not pathogenic to piglets and can induce shnAbs against both G2 PEDV and HP-PRRSV2 isolates after prime-boost immunization. However, passive transfer study in neonatal piglets deprived of sow colostrum showed that rJSTZ1712-12-S-induced shnAbs may only decrease PEDV and PRRSV viremia but cannot confer sufficient protection against dual challenge of high virulent G2 PEDV XJ1904-34 strain and HP-PRRSV2 XJ17-5 isolate. Overall, this study provides the first evidence that shnAbs confer insufficient protection against PEDV and PRRSV coinfection and are inadequate for the evaluation of protective efficacy of PED and PRRS bivalent vaccine (especially for the PED vaccine). IMPORTANCE Porcine epidemic diarrhea virus (PEDV) and porcine reproductive and respiratory syndrome virus (PRRSV) coinfection occurs commonly and can synergistically reduce feed intake and pig growth. Vaccination is an effective strategy utilized for PED and PRRS control, and systemic homologous neutralizing antibodies (shnAbs) in serum are commonly used for protective efficacy evaluation of PED and PRRS vaccines. Currently, no commercial vaccine is available against PEDV and PRRSV coinfection. This study generated a chimeric vaccine candidate against the coinfection of prevalent PEDV and PRRSV in China. The chimeric strain can induce satisfied shnAbs against both PEDV and PRRSV after prime-boost inoculation in pigs. But the shnAbs cannot confer sufficient protection against PEDV and PRRSV coinfection in neonatal piglets. To the best of our knowledge, these findings provide the first evidence that shnAbs confer insufficient protection against PEDV and PRRSV coinfection and are inadequate for evaluating PED and PRRS bivalent vaccine protective efficacy.
Keywords: chimeric vaccine; neonatal piglet; porcine epidemic diarrhea virus (PEDV); porcine reproductive and respiratory syndrome virus (PRRSV); protective efficacy; systemic homologous neutralizing antibodies (shnAbs).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Construction and characterization of a gE/gI/TK-gene-deleted recombinant pseudorabies virus variant expressing the GP5 of the highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) and NADC30-like PRRSV.Microb Pathog. 2025 Jun;203:107522. doi: 10.1016/j.micpath.2025.107522. Epub 2025 Apr 1. Microb Pathog. 2025. PMID: 40180235
-
Minor and major envelope proteins of PRRSV play synergistic roles in inducing heterologous neutralizing antibodies and conferring cross protection.Virus Res. 2022 Jul 2;315:198789. doi: 10.1016/j.virusres.2022.198789. Epub 2022 Apr 26. Virus Res. 2022. PMID: 35487365
-
Chimeric HP-PRRSV2 containing an ORF2-6 consensus sequence induces antibodies with broadly neutralizing activity and confers cross protection against virulent NADC30-like isolate.Vet Res. 2021 May 27;52(1):74. doi: 10.1186/s13567-021-00944-8. Vet Res. 2021. PMID: 34044890 Free PMC article.
-
Live porcine reproductive and respiratory syndrome virus vaccines: Current status and future direction.Vaccine. 2015 Aug 7;33(33):4069-80. doi: 10.1016/j.vaccine.2015.06.092. Epub 2015 Jul 4. Vaccine. 2015. PMID: 26148878 Review.
-
Innate and adaptive immunity against Porcine Reproductive and Respiratory Syndrome Virus.Vet Immunol Immunopathol. 2015 Sep 15;167(1-2):1-14. doi: 10.1016/j.vetimm.2015.07.003. Epub 2015 Jul 17. Vet Immunol Immunopathol. 2015. PMID: 26209116 Free PMC article. Review.
Cited by
-
Pathogenicity Studies of NADC34-like Porcine Reproductive and Respiratory Syndrome Virus LNSY-GY and NADC30-like Porcine Reproductive and Respiratory Syndrome Virus GXGG-8011 in Piglets.Viruses. 2023 Nov 11;15(11):2247. doi: 10.3390/v15112247. Viruses. 2023. PMID: 38005924 Free PMC article.
-
A chimeric porcine reproductive and respiratory syndrome virus 1 strain containing synthetic ORF2-6 genes can trigger T follicular helper cell and heterologous neutralizing antibody responses and confer enhanced cross-protection.Vet Res. 2024 Mar 6;55(1):28. doi: 10.1186/s13567-024-01280-3. Vet Res. 2024. PMID: 38449049 Free PMC article.
-
Propidium Monoazide Integrated With qPCR Enables Rapid and Universal Detection of Infectious Porcine Reproductive and Respiratory Syndrome Viruses.Transbound Emerg Dis. 2024 Dec 21;2024:6250851. doi: 10.1155/tbed/6250851. eCollection 2024. Transbound Emerg Dis. 2024. PMID: 40303043 Free PMC article.
-
Genetic characterization and pathogenicity analysis of three porcine epidemic diarrhea virus strains isolated from North China.Vet Res. 2025 Jun 14;56(1):118. doi: 10.1186/s13567-025-01554-4. Vet Res. 2025. PMID: 40517255 Free PMC article.
-
Advances in porcine epidemic diarrhea virus research: genome, epidemiology, vaccines, and detection methods.Discov Nano. 2025 Mar 3;20(1):48. doi: 10.1186/s11671-025-04220-y. Discov Nano. 2025. PMID: 40029472 Free PMC article. Review.
References
-
- Chen N, Li S, Tian Y, Li X, Li S, Li J, Qiu M, Sun Z, Xiao Y, Yan X, Lin H, Yu X, Tian K, Shang S, Zhu J. 2021. Chimeric HP-PRRSV2 containing an ORF2-6 consensus sequence induces antibodies with broadly neutralizing activity and confers cross protection against virulent NADC30-like isolate. Vet Res 52:74. doi:10.1186/s13567-021-00944-8. - DOI - PMC - PubMed
-
- Xuan H, Xing D, Wang D, Zhu W, Zhao F, Gong H. 1984. Study on the culture of porcine epidemic diarrhea virus adapted to fetal porcine intestine primary cell monolayer. Chin J Vet Sci 4:202–208.
-
- Guo B, Chen Z, Liu W, Cui Y. 1996. Isolation and identification of porcine reproductive and respiratory syndrome (PRRS) virus from aborted fetuses suspected of PRRS. Chin J Prev Vet Med 2:1–5.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

