Trends in Use of Single- vs Dual-Chamber Implantable Cardioverter-Defibrillators Among Patients Without a Pacing Indication, 2010-2018
- PMID: 35315917
- PMCID: PMC8941353
- DOI: 10.1001/jamanetworkopen.2022.3429
Trends in Use of Single- vs Dual-Chamber Implantable Cardioverter-Defibrillators Among Patients Without a Pacing Indication, 2010-2018
Abstract
Importance: Use of dual-chamber implantable cardioverter-defibrillator (ICD) systems among patients without a pacing indication is an example of low-value care given higher procedural risks, higher costs, and little evidence for benefit from an atrial lead. However, variation in the use of dual-chamber systems was present among patients without a pacing indication.
Objective: To examine the temporal trends and hospital variation in use of single- and dual-chamber ICD implantation among patients without a pacing indication undergoing first-time ICD implantation.
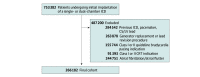
Design, setting, and participants: A multicenter cross-sectional study was conducted using the US National Cardiovascular Data Registry ICD Registry. A total of 266 182 patients undergoing initial implantation of a single- or dual-chamber transvenous ICD without a bradycardia pacing indication, class I or II cardiac resynchronization therapy indication, or history of atrial fibrillation or atrial flutter were included. The study was conducted from April 1, 2010, to December 31, 2018; data analysis was performed from October 19, 2020, to January 5, 2022.
Exposures: Implantation of a single- or dual-chamber ICD.
Main outcomes and measures: Temporal trends among patients undergoing single- vs dual-chamber ICDs were determined using the Cochran-Armitage trend test, and hospital-level variation using adjusted hospital median odds ratios was examined.
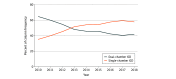
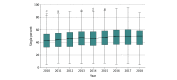
Results: A total of 266 182 patients (single-chamber ICD, 134 925; dual-chamber ICD, 131 257) were included in this analysis; mean (SD) age was 58.0 (14.0) years and 91 990 patients (68.2%) were men. The use of dual-chamber ICDs decreased from 64.7% (n = 15 694) in 2010 to 42.2% (n = 9762) in 2018 (P < .001). Adjusted for patient characteristics, the median hospital-level proportion of single-chamber ICDs increased from 42.9% (95% CI, 42.6%-45.0%) in 2010 to 50.0% (95% CI, 47.8%-51.0%) in 2018. The median odds ratio for the use of dual-chamber ICDs, adjusted for patient characteristics, was 1.6 (95% CI, 1.6-1.8) in 2010 and 1.5 (95% CI, 1.5-1.8) in 2018, indicating decreasing but persistent variation in use.
Conclusions and relevance: In this national study of US patients undergoing first-time ICD implantation without a clinical indication for an atrial lead, the use of dual-chamber devices decreased. However, institutional variability in the use of atrial leads persists, suggesting differences in individual or institutional cultures of real-world practice and opportunity to reduce this low-value practice.
Conflict of interest statement
Figures
References
-
- Moss AJ, Zareba W, Hall WJ, et al. ; Multicenter Automatic Defibrillator Implantation Trial II Investigators . Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877-883. doi:10.1056/NEJMoa013474 - DOI - PubMed
-
- Epstein AE, DiMarco JP, Ellenbogen KA, et al. ; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Heart Rhythm Society . 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2013;61(3):e6-e75. doi:10.1016/j.jacc.2012.11.007 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

