Protein synthesis control in cancer: selectivity and therapeutic targeting
- PMID: 35315941
- PMCID: PMC9016353
- DOI: 10.15252/embj.2021109823
Protein synthesis control in cancer: selectivity and therapeutic targeting
Abstract
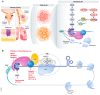
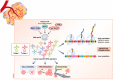
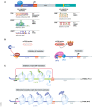
Translational control of mRNAs is a point of convergence for many oncogenic signals through which cancer cells tune protein expression in tumorigenesis. Cancer cells rely on translational control to appropriately adapt to limited resources while maintaining cell growth and survival, which creates a selective therapeutic window compared to non-transformed cells. In this review, we first discuss how cancer cells modulate the translational machinery to rapidly and selectively synthesize proteins in response to internal oncogenic demands and external factors in the tumor microenvironment. We highlight the clinical potential of compounds that target different translation factors as anti-cancer therapies. Next, we detail how RNA sequence and structural elements interface with the translational machinery and RNA-binding proteins to coordinate the translation of specific pro-survival and pro-growth programs. Finally, we provide an overview of the current and emerging technologies that can be used to illuminate the mechanisms of selective translational control in cancer cells as well as within the microenvironment.
Keywords: cancer; protein synthesis; translation and protein quality; translation inhibitors; translational control.
© 2022 The Authors.
Figures


References
-
- Archer SK, Shirokikh NE, Beilharz TH, Preiss T (2016) Dynamics of ribosome scanning and recycling revealed by translation complex profiling. Nature 535: 570–574 - PubMed
-
- Attar‐Schneider O, Drucker L, Zismanov V, Tartakover‐Matalon S, Lishner M (2014) Targeting eIF4GI translation initiation factor affords an attractive therapeutic strategy in multiple myeloma. Cell Signal 26: 1878–1887 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

