How bacteria utilize sialic acid during interactions with the host: snip, snatch, dispatch, match and attach
- PMID: 35316172
- PMCID: PMC9558349
- DOI: 10.1099/mic.0.001157
How bacteria utilize sialic acid during interactions with the host: snip, snatch, dispatch, match and attach
Abstract
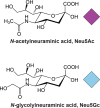
N -glycolylneuraminic acid (Neu5Gc), and its precursor N-acetylneuraminic acid (Neu5Ac), commonly referred to as sialic acids, are two of the most common glycans found in mammals. Humans carry a mutation in the enzyme that converts Neu5Ac into Neu5Gc, and as such, expression of Neu5Ac can be thought of as a 'human specific' trait. Bacteria can utilize sialic acids as a carbon and energy source and have evolved multiple ways to take up sialic acids. In order to generate free sialic acid, many bacteria produce sialidases that cleave sialic acid residues from complex glycan structures. In addition, sialidases allow escape from innate immune mechanisms, and can synergize with other virulence factors such as toxins. Human-adapted pathogens have evolved a preference for Neu5Ac, with many bacterial adhesins, and major classes of toxin, specifically recognizing Neu5Ac containing glycans as receptors. The preference of human-adapted pathogens for Neu5Ac also occurs during biosynthesis of surface structures such as lipo-oligosaccharide (LOS), lipo-polysaccharide (LPS) and polysaccharide capsules, subverting the human host immune system by mimicking the host. This review aims to provide an update on the advances made in understanding the role of sialic acid in bacteria-host interactions made in the last 5-10 years, and put these findings into context by highlighting key historical discoveries. We provide a particular focus on 'molecular mimicry' and incorporation of sialic acid onto the bacterial outer-surface, and the role of sialic acid as a receptor for bacterial adhesins and toxins.
Keywords: N-acetylneuraminic acid; N-glycolylneuraminic acid; Neu5Ac; Neu5GC; adherence; adhesin; bacterial pathogen; molecular mimicry; sialic acid.
Conflict of interest statement
The authors declare that there are no conflicts of interest
Figures
Similar articles
-
Nontypeable Haemophilus influenzae Has Evolved Preferential Use of N-Acetylneuraminic Acid as a Host Adaptation.mBio. 2019 May 7;10(3):e00422-19. doi: 10.1128/mBio.00422-19. mBio. 2019. PMID: 31064827 Free PMC article.
-
Exploring the Impact of Ketodeoxynonulosonic Acid in Host-Pathogen Interactions Using Uptake and Surface Display by Nontypeable Haemophilus influenzae.mBio. 2021 Jan 19;12(1):e03226-20. doi: 10.1128/mBio.03226-20. mBio. 2021. PMID: 33468699 Free PMC article.
-
Biosynthesis of N-glycolyneuraminic acid. The primary site of hydroxylation of N-acetylneuraminic acid is the cytosolic sugar nucleotide pool.J Biol Chem. 1989 Dec 5;264(34):20216-23. J Biol Chem. 1989. PMID: 2684973
-
Sialic acid diversity in the human gut: Molecular impacts and tools for future discovery.Curr Opin Struct Biol. 2022 Aug;75:102397. doi: 10.1016/j.sbi.2022.102397. Epub 2022 May 30. Curr Opin Struct Biol. 2022. PMID: 35653953 Review.
-
N-glycolylneuraminic acid conjugates: implications of their absence in mammalian biochemistry.Indian J Biochem Biophys. 2003 Aug;40(4):217-25. Indian J Biochem Biophys. 2003. PMID: 22900313 Review.
Cited by
-
Role of Xenosialylation in Post-Infectious and Post-Vaccination Complications, Including Covid-19 and Anti-SARS-CoV-2 Vaccination.J Inflamm Res. 2024 Nov 7;17:8385-8394. doi: 10.2147/JIR.S471093. eCollection 2024. J Inflamm Res. 2024. PMID: 39529999 Free PMC article.
-
Phage-encoded depolymerases as a strategy for combating multidrug-resistant Acinetobacter baumannii.Front Cell Infect Microbiol. 2024 Oct 24;14:1462620. doi: 10.3389/fcimb.2024.1462620. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39512587 Free PMC article. Review.
-
Glycosphingolipids: from metabolism to chemoenzymatic total synthesis.Org Biomol Chem. 2024 Aug 22;22(33):6665-6683. doi: 10.1039/d4ob00695j. Org Biomol Chem. 2024. PMID: 39120686 Free PMC article. Review.
-
Nutritional Interactions between Bacterial Species Colonising the Human Nasal Cavity: Current Knowledge and Future Prospects.Metabolites. 2022 May 27;12(6):489. doi: 10.3390/metabo12060489. Metabolites. 2022. PMID: 35736422 Free PMC article. Review.
-
Many locks to one key: N-acetylneuraminic acid binding to proteins.IUCrJ. 2024 Sep 1;11(Pt 5):664-674. doi: 10.1107/S2052252524005360. IUCrJ. 2024. PMID: 38965900 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

