ADAMTS2 and ADAMTS14 can substitute for ADAMTS3 in adults for pro-VEGFC activation and lymphatic homeostasis
- PMID: 35316211
- PMCID: PMC9089798
- DOI: 10.1172/jci.insight.151509
ADAMTS2 and ADAMTS14 can substitute for ADAMTS3 in adults for pro-VEGFC activation and lymphatic homeostasis
Abstract
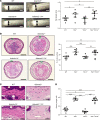
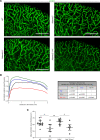
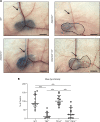
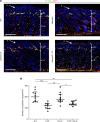
The capacity of ADAMTS3 to cleave pro-VEGFC into active VEGFC able to bind its receptors and to stimulate lymphangiogenesis has been clearly established during embryonic life. However, this function of ADAMTS3 is unlikely to persist in adulthood because of its restricted expression pattern after birth. Because ADAMTS2 and ADAMTS14 are closely related to ADAMTS3 and are mainly expressed in connective tissues where the lymphatic network extends, we hypothesized that they could substitute for ADAMTS3 during adulthood in mammals allowing proteolytic activation of pro-VEGFC. Here, we demonstrated that ADAMTS2 and ADAMTS14 are able to process pro-VEGFC into active VEGFC as efficiently as ADAMTS3. In vivo, adult mice lacking Adamts2 developed skin lymphedema due to a reduction of the density and diameter of lymphatic vessels, leading to a decrease of lymphatic functionality, while genetic ablation of Adamts14 had no impact. In a model of thermal cauterization of cornea, lymphangiogenesis was significantly reduced in Adamts2- and Adamts14-KO mice and further repressed in Adamts2/Adamts14 double-KO mice. In summary, we have demonstrated that ADAMTS2 and ADAMTS14 are as efficient as ADAMTS3 in activation of pro-VEGFC and are involved in the homeostasis of the lymphatic vasculature in adulthood, both in physiological and pathological processes.
Keywords: Angiogenesis; Cardiovascular disease; Growth factors; Vascular Biology.
Figures


Similar articles
-
Proteolytic activation defines distinct lymphangiogenic mechanisms for VEGFC and VEGFD.J Clin Invest. 2016 Jun 1;126(6):2167-80. doi: 10.1172/JCI83967. Epub 2016 May 9. J Clin Invest. 2016. PMID: 27159393 Free PMC article.
-
Specific fibroblast subpopulations and neuronal structures provide local sources of Vegfc-processing components during zebrafish lymphangiogenesis.Nat Commun. 2020 Jun 1;11(1):2724. doi: 10.1038/s41467-020-16552-7. Nat Commun. 2020. PMID: 32483144 Free PMC article.
-
Spontaneous atopic dermatitis due to immune dysregulation in mice lacking Adamts2 and 14.Matrix Biol. 2018 Sep;70:140-157. doi: 10.1016/j.matbio.2018.04.002. Epub 2018 Apr 9. Matrix Biol. 2018. PMID: 29649548
-
Molecular biology and pathology of lymphangiogenesis.Annu Rev Pathol. 2008;3:367-97. doi: 10.1146/annurev.pathmechdis.3.121806.151515. Annu Rev Pathol. 2008. PMID: 18039141 Review.
-
Lymphatic vascular morphogenesis in development, physiology, and disease.J Cell Biol. 2011 May 16;193(4):607-18. doi: 10.1083/jcb.201012094. J Cell Biol. 2011. PMID: 21576390 Free PMC article. Review.
Cited by
-
ADAMTS2: More than a procollagen N-proteinase.Genes Dis. 2025 May 13;12(6):101686. doi: 10.1016/j.gendis.2025.101686. eCollection 2025 Nov. Genes Dis. 2025. PMID: 40837410 Free PMC article. Review.
-
Current Status of Lymphangiogenesis: Molecular Mechanism, Immune Tolerance, and Application Prospect.Cancers (Basel). 2023 Feb 11;15(4):1169. doi: 10.3390/cancers15041169. Cancers (Basel). 2023. PMID: 36831512 Free PMC article. Review.
-
Lymphatic vessel: origin, heterogeneity, biological functions, and therapeutic targets.Signal Transduct Target Ther. 2024 Jan 3;9(1):9. doi: 10.1038/s41392-023-01723-x. Signal Transduct Target Ther. 2024. PMID: 38172098 Free PMC article. Review.
-
Large scale phenotype imputation and in vivo functional validation implicate ADAMTS14 as an adiposity gene.Nat Commun. 2023 Jan 19;14(1):307. doi: 10.1038/s41467-022-35563-0. Nat Commun. 2023. PMID: 36658113 Free PMC article.
-
Novel Homozygous ADAMTS2 Variants and Associated Disease Phenotypes in Dogs with Dermatosparactic Ehlers-Danlos Syndrome.Genes (Basel). 2022 Nov 19;13(11):2158. doi: 10.3390/genes13112158. Genes (Basel). 2022. PMID: 36421833 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

