Aerobic exercise elicits clinical adaptations in myotonic dystrophy type 1 patients independently of pathophysiological changes
- PMID: 35316212
- PMCID: PMC9106360
- DOI: 10.1172/JCI156125
Aerobic exercise elicits clinical adaptations in myotonic dystrophy type 1 patients independently of pathophysiological changes
Abstract
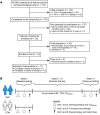
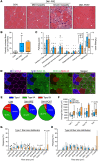
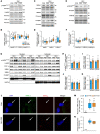
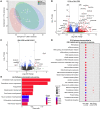
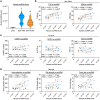
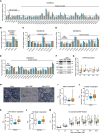
BackgroundMyotonic dystrophy type 1 (DM1) is a complex life-limiting neuromuscular disorder characterized by severe skeletal muscle atrophy, weakness, and cardiorespiratory defects. Exercised DM1 mice exhibit numerous physiological benefits that are underpinned by reduced CUG foci and improved alternative splicing. However, the efficacy of physical activity in patients is unknown.MethodsEleven genetically diagnosed DM1 patients were recruited to examine the extent to which 12 weeks of cycling can recuperate clinical and physiological metrics. Furthermore, we studied the underlying molecular mechanisms through which exercise elicits benefits in skeletal muscle of DM1 patients.RESULTSDM1 was associated with impaired muscle function, fitness, and lung capacity. Cycling evoked several clinical, physical, and metabolic advantages in DM1 patients. We highlight that exercise-induced molecular and cellular alterations in patients do not conform with previously published data in murine models and propose a significant role of mitochondrial function in DM1 pathology. Finally, we discovered a subset of small nucleolar RNAs (snoRNAs) that correlated to indicators of disease severity.ConclusionWith no available cures, our data support the efficacy of exercise as a primary intervention to partially mitigate the clinical progression of DM1. Additionally, we provide evidence for the involvement of snoRNAs and other noncoding RNAs in DM1 pathophysiology.Trial registrationThis trial was approved by the HiREB committee (no. 7901) and registered under ClinicalTrials.gov (NCT04187482).FundingNeil and Leanne Petroff. Canadian Institutes of Health Research Foundation (no. 143325).
Keywords: Cell Biology; Mitochondria; Muscle Biology; Neuromuscular disease; RNA processing.
Conflict of interest statement
Figures


Comment in
-
Benefits of aerobic exercise in myotonic dystrophy type 1.J Clin Invest. 2022 May 16;132(10):e160229. doi: 10.1172/JCI160229. J Clin Invest. 2022. PMID: 35575095 Free PMC article.
Similar articles
-
Aerobic Training Alleviates Muscle Atrophy by Promoting the Proliferation of Skeletal Muscle Satellite Cells in Myotonic Dystrophy Type 1 by Inhibiting Glycolysis via the Upregulation of MBNL1.Curr Stem Cell Res Ther. 2025;20(4):449-463. doi: 10.2174/011574888X360503241214045130. Curr Stem Cell Res Ther. 2025. PMID: 39710919
-
Chronic exercise mitigates disease mechanisms and improves muscle function in myotonic dystrophy type 1 mice.J Physiol. 2019 Mar;597(5):1361-1381. doi: 10.1113/JP277123. Epub 2019 Jan 30. J Physiol. 2019. PMID: 30628727 Free PMC article.
-
A single dose of exercise stimulates skeletal muscle mitochondrial plasticity in myotonic dystrophy type 1.Acta Physiol (Oxf). 2023 Apr;237(4):e13943. doi: 10.1111/apha.13943. Epub 2023 Feb 10. Acta Physiol (Oxf). 2023. PMID: 36726043
-
What is known about the effects of exercise or training to reduce skeletal muscle impairments of patients with myotonic dystrophy type 1? A scoping review.BMC Musculoskelet Disord. 2019 Mar 5;20(1):101. doi: 10.1186/s12891-019-2458-7. BMC Musculoskelet Disord. 2019. PMID: 30836978 Free PMC article.
-
Molecular mechanisms of muscle atrophy in myotonic dystrophies.Int J Biochem Cell Biol. 2013 Oct;45(10):2280-7. doi: 10.1016/j.biocel.2013.06.010. Epub 2013 Jun 21. Int J Biochem Cell Biol. 2013. PMID: 23796888 Free PMC article. Review.
Cited by
-
Effect of exercise training on clinical and physiological variables in adults with myotonic dystrophy type 1: A systematic review protocol.MethodsX. 2024 Sep 12;13:102957. doi: 10.1016/j.mex.2024.102957. eCollection 2024 Dec. MethodsX. 2024. PMID: 39376683 Free PMC article.
-
A Novel Class of FKBP12 Ligands Rescues Premature Aging Phenotypes Associated with Myotonic Dystrophy Type 1.Cells. 2024 Nov 22;13(23):1939. doi: 10.3390/cells13231939. Cells. 2024. PMID: 39682688 Free PMC article.
-
Energy Expenditure, Body Composition, and Skeletal Muscle Oxidative Capacity in Patients with Myotonic Dystrophy Type 1.J Neuromuscul Dis. 2023;10(4):701-712. doi: 10.3233/JND-230036. J Neuromuscul Dis. 2023. PMID: 37154183 Free PMC article.
-
Myotonic Dystrophy type 2 unmasked by physical activity resumption following COVID-19 lockdown: case discussion and review of the literature.Acta Myol. 2024 Dec;43(4):134-138. doi: 10.36185/2532-1900-612. Acta Myol. 2024. PMID: 40017289 Free PMC article. Review.
-
Aerobic Training Alleviates Muscle Atrophy by Promoting the Proliferation of Skeletal Muscle Satellite Cells in Myotonic Dystrophy Type 1 by Inhibiting Glycolysis via the Upregulation of MBNL1.Curr Stem Cell Res Ther. 2025;20(4):449-463. doi: 10.2174/011574888X360503241214045130. Curr Stem Cell Res Ther. 2025. PMID: 39710919
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

