Iron deficiency linked to altered bile acid metabolism promotes Helicobacter pylori-induced inflammation-driven gastric carcinogenesis
- PMID: 35316215
- PMCID: PMC9106351
- DOI: 10.1172/JCI147822
Iron deficiency linked to altered bile acid metabolism promotes Helicobacter pylori-induced inflammation-driven gastric carcinogenesis
Abstract
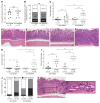
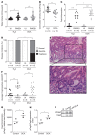
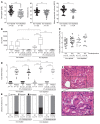
Gastric carcinogenesis is mediated by complex interactions among Helicobacter pylori, host, and environmental factors. Here, we demonstrate that H. pylori augmented gastric injury in INS-GAS mice under iron-deficient conditions. Mechanistically, these phenotypes were not driven by alterations in the gastric microbiota; however, discovery-based and targeted metabolomics revealed that bile acids were significantly altered in H. pylori-infected mice with iron deficiency, with significant upregulation of deoxycholic acid (DCA), a carcinogenic bile acid. The severity of gastric injury was further augmented when H. pylori-infected mice were treated with DCA, and, in vitro, DCA increased translocation of the H. pylori oncoprotein CagA into host cells. Conversely, bile acid sequestration attenuated H. pylori-induced injury under conditions of iron deficiency. To translate these findings to human populations, we evaluated the association between bile acid sequestrant use and gastric cancer risk in a large human cohort. Among 416,885 individuals, a significant dose-dependent reduction in risk was associated with cumulative bile acid sequestrant use. Further, expression of the bile acid receptor transmembrane G protein-coupled bile acid receptor 5 (TGR5) paralleled the severity of carcinogenic lesions in humans. These data demonstrate that increased H. pylori-induced injury within the context of iron deficiency is tightly linked to altered bile acid metabolism, which may promote gastric carcinogenesis.
Keywords: Bacterial infections; Gastric cancer; Gastroenterology; Infectious disease; Mouse models.
Conflict of interest statement
Figures





Comment in
-
Gastrointestinal neoplasia: carcinogenic interaction between bile acids and Helicobacter pylori in the stomach.J Clin Invest. 2022 May 16;132(10):e160194. doi: 10.1172/JCI160194. J Clin Invest. 2022. PMID: 35575088 Free PMC article.
Similar articles
-
IRON DEFICIENCY PROMOTES HELICOBACTER PYLORI-INDUCED GASTRIC CARCINOGENESIS BY ENABLING RECIPROCITY BETWEEN CARCINOGENIC SECONDARY BILE ACIDS AND PROTECTIVE LONG-CHAIN FATTY ACIDS.Trans Am Clin Climatol Assoc. 2025;135:206-221. Trans Am Clin Climatol Assoc. 2025. PMID: 40771613 Free PMC article.
-
Modification of the Gastric Mucosal Microbiota by a Strain-Specific Helicobacter pylori Oncoprotein and Carcinogenic Histologic Phenotype.mBio. 2019 May 28;10(3):e00955-19. doi: 10.1128/mBio.00955-19. mBio. 2019. PMID: 31138752 Free PMC article.
-
CagA mediates epigenetic regulation to attenuate let-7 expression in Helicobacter pylori-related carcinogenesis.Gut. 2013 Nov;62(11):1536-46. doi: 10.1136/gutjnl-2011-301625. Epub 2012 Aug 30. Gut. 2013. PMID: 22936674
-
Molecular Pathogenesis of Helicobacter pylori-Related Gastric Cancer.Gastroenterol Clin North Am. 2015 Sep;44(3):625-38. doi: 10.1016/j.gtc.2015.05.011. Epub 2015 Jul 7. Gastroenterol Clin North Am. 2015. PMID: 26314672 Review.
-
Role of Helicobacter pylori virulence factor cytotoxin-associated gene A in gastric mucosa-associated lymphoid tissue lymphoma.World J Gastroenterol. 2013 Dec 7;19(45):8219-26. doi: 10.3748/wjg.v19.i45.8219. World J Gastroenterol. 2013. PMID: 24363512 Free PMC article. Review.
Cited by
-
Bile Acid Sequestrant Use and Gastric Cancer: A National Retrospective Cohort Analysis.Clin Transl Gastroenterol. 2023 Dec 1;14(12):e00596. doi: 10.14309/ctg.0000000000000596. Clin Transl Gastroenterol. 2023. PMID: 37606521 Free PMC article.
-
Exploring changes in metabolites and fecal microbiota of advanced gastric cancer based on plasma metabolomics and 16S rDNA sequencing.Heliyon. 2025 Jan 6;11(2):e41715. doi: 10.1016/j.heliyon.2025.e41715. eCollection 2025 Jan 30. Heliyon. 2025. PMID: 39897929 Free PMC article.
-
Helicobacter pylori infection induces DNA double-strand breaks through the ACVR1/IRF3/POLD1 signaling axis to drive gastric tumorigenesis.Gut Microbes. 2025 Dec;17(1):2463581. doi: 10.1080/19490976.2025.2463581. Epub 2025 Feb 9. Gut Microbes. 2025. PMID: 39924917 Free PMC article.
-
Ferroptosis: opening up potential targets for gastric cancer treatment.Mol Cell Biochem. 2024 Nov;479(11):2863-2874. doi: 10.1007/s11010-023-04886-x. Epub 2023 Dec 11. Mol Cell Biochem. 2024. PMID: 38082184 Review.
-
Distinctive duodenal microbiomes and bile acid profiles in duodenal tumor patients revealed by prospective observational study.Sci Rep. 2024 Aug 12;14(1):18705. doi: 10.1038/s41598-024-69820-7. Sci Rep. 2024. PMID: 39134638 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

