A multitope SARS-CoV-2 vaccine provides long-lasting B cell and T cell immunity against Delta and Omicron variants
- PMID: 35316221
- PMCID: PMC9106357
- DOI: 10.1172/JCI157707
A multitope SARS-CoV-2 vaccine provides long-lasting B cell and T cell immunity against Delta and Omicron variants
Abstract
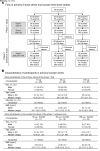
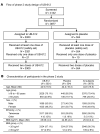
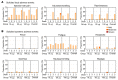
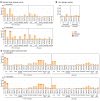
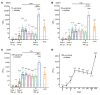
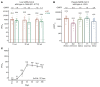
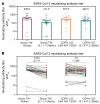
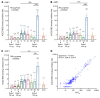
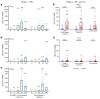
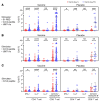
BackgroundThe Delta and Omicron variants of SARS-CoV-2 are currently responsible for breakthrough infections due to waning immunity. We report phase I/II trial results of UB-612, a multitope subunit vaccine containing S1-RBD-sFc protein and rationally designed promiscuous peptides representing sarbecovirus conserved helper T cell and cytotoxic T lymphocyte epitopes on the nucleocapsid (N), membrane (M), and spike (S2) proteins.MethodWe conducted a phase I primary 2-dose (28 days apart) trial of 10, 30, or 100 μg UB-612 in 60 healthy young adults 20 to 55 years old, and 50 of them were boosted with 100 μg of UB-612 approximately 7 to 9 months after the second dose. A separate placebo-controlled and randomized phase II study was conducted with 2 doses of 100 μg of UB-612 (n = 3,875, 18-85 years old). We evaluated interim safety and immunogenicity of phase I until 14 days after the third (booster) dose and of phase II until 28 days after the second dose.ResultsNo vaccine-related serious adverse events were recorded. The most common solicited adverse events were injection site pain and fatigue, mostly mild and transient. In both trials, UB-612 elicited respective neutralizing antibody titers similar to a panel of human convalescent sera. The most striking findings were long-lasting virus-neutralizing antibodies and broad T cell immunity against SARS-CoV-2 variants of concern (VoCs), including Delta and Omicron, and a strong booster-recalled memory immunity with high cross-reactive neutralizing titers against the Delta and Omicron VoCs.ConclusionUB-612 has presented a favorable safety profile, potent booster effect against VoCs, and long-lasting B and broad T cell immunity that warrants further development for both primary immunization and heterologous boosting of other COVID-19 vaccines.Trial RegistrationClinicalTrials.gov: NCT04545749, NCT04773067, and NCT04967742.FundingUBI Asia, Vaxxinity Inc., and Taiwan Centers for Disease Control, Ministry of Health and Welfare.
Keywords: COVID-19; Peptides.
Conflict of interest statement
Figures
References
-
- Suthar MS, et al. Durability of immune responses to the BNT162b2 mRNA vaccine [preprint]. Posted on bioRxiv September 30, 2021. - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

