Changes in Cervical Cytology Results and Human Papillomavirus Types Among Persons Screened for Cervical Cancer, 2007 and 2015-2017
- PMID: 35316258
- PMCID: PMC8972086
- DOI: 10.1097/LGT.0000000000000659
Changes in Cervical Cytology Results and Human Papillomavirus Types Among Persons Screened for Cervical Cancer, 2007 and 2015-2017
Abstract
Objectives: Since 2006, the US human papillomavirus (HPV) vaccination program has led to decreases in HPV infections caused by high-risk vaccine-targeted HPV types (HPV 16/18). We assessed differences in high-risk HPV prevalence by cervical cytology result among 20- to 24-year-old persons participating in routine cervical cancer screening in 2015-2017 compared with 2007.
Materials and methods: Residual routine cervical cancer screening specimens were collected from 20- to 24-year-old members of 2 integrated healthcare delivery systems as part of a cross-sectional study and were tested for 37 HPV types. Cytology results and vaccination status (≥1 dose) were extracted from medical records. Cytology categories were normal, atypical squamous cells of undefined significance, low-grade squamous intraepithelial lesions (SIL), or high-grade SIL/atypical squamous cells cannot exclude high-grade SIL. Prevalences of HPV categories (HPV 16/18, HPV 31/33/45/52/58, HPV 35/39/51/56/59/66/68) were estimated by cytology result for 2007 and 2015-2017.
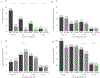
Results: Specimens from 2007 (n = 4046) were from unvaccinated participants; 4574 of 8442 specimens (54.2%) from 2015-2017 were from vaccinated participants. Overall, HPV 16/18 positivity was lower in 2015-2017 compared with 2007 in all groups: high-grade SIL/atypical squamous cells cannot exclude high-grade SIL, 16.0% vs 69.2%; low-grade SIL, 5.4% vs 40.1%; atypical squamous cells of undefined significance, 5.0% vs 25.6%; and normal, 1.3% vs 8.1%. Human papillomavirus 31/33/45/52/58 prevalence was stable for all cytology groups; HPV 35/39/51/56/59/66/68 prevalence increased among low-grade SIL specimens (53.9% to 65.2%) but remained stable in other groups.
Conclusions: Prevalence of vaccine-targeted high-risk HPV types 16/18 was dramatically lower in 2015-2017 than 2007 across all cytology result groups while prevalence of other high-risk HPV types was mainly stable, supporting vaccine impact with no evidence of type replacement.
Copyright © 2022, ASCCP.
Conflict of interest statement
A.L.N. has received research funding from Pfizer, Takeda, MedImmune, and Merck for unrelated studies. N.P.K. has received research funding from Merck, GlaxoSmithKline, Sanofi Pasteur, Novartis, MedImmune, Pfizer, and Protein Sciences (now Sanofi Pasteur) for unrelated studies. S.W. has received research funding from GlaxoSmithKline for an unrelated study. The other authors have declared they have no conflicts of interest.
Figures
References
-
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. Mar 23 2007;56(Rr-2):1–24. - PubMed
-
- Rosenblum HG, Lewis RM, Gargano JW, Querec TD, Unger ER, Markowitz LE. Declines in prevalence of human papillomavirus vaccine-type infection among females after introduction of vaccine - United States, 2003–2018. MMWR Morb Mortal Wkly Rep. Mar 26 2021;70(12):415–420. doi:10.15585/mmwr.mm7012a2 - DOI - PMC - PubMed

