Sustained chemogenetic activation of locus coeruleus norepinephrine neurons promotes dopaminergic neuron survival in synucleinopathy
- PMID: 35316276
- PMCID: PMC8939823
- DOI: 10.1371/journal.pone.0263074
Sustained chemogenetic activation of locus coeruleus norepinephrine neurons promotes dopaminergic neuron survival in synucleinopathy
Abstract
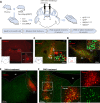
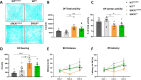
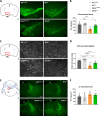
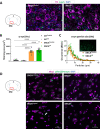
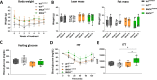
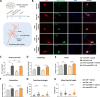
Dopaminergic neuron degeneration in the midbrain plays a pivotal role in motor symptoms associated with Parkinson's disease. However, non-motor symptoms of Parkinson's disease and post-mortem histopathology confirm dysfunction in other brain areas, including the locus coeruleus and its associated neurotransmitter norepinephrine. Here, we investigate the role of central norepinephrine-producing neurons in Parkinson's disease by chronically stimulating catecholaminergic neurons in the locus coeruleus using chemogenetic manipulation. We show that norepinephrine neurons send complex axonal projections to the dopaminergic neurons in the substantia nigra, confirming physical communication between these regions. Furthermore, we demonstrate that increased activity of norepinephrine neurons is protective against dopaminergic neuronal depletion in human α-syn A53T missense mutation over-expressing mice and prevents motor dysfunction in these mice. Remarkably, elevated norepinephrine neurons action fails to alleviate α-synuclein aggregation and microgliosis in the substantia nigra suggesting the presence of an alternate neuroprotective mechanism. The beneficial effects of high norepinephrine neuron activity might be attributed to the action of norepinephrine on dopaminergic neurons, as recombinant norepinephrine treatment increased primary dopaminergic neuron cultures survival and neurite sprouting. Collectively, our results suggest a neuroprotective mechanism where noradrenergic neurons activity preserves the integrity of dopaminergic neurons, which prevents synucleinopathy-dependent loss of these cells.
Conflict of interest statement
The authors declare no competing interest.
Figures
Similar articles
-
Intracerebral Administration of a Ligand-ASO Conjugate Selectively Reduces α-Synuclein Accumulation in Monoamine Neurons of Double Mutant Human A30P*A53T*α-Synuclein Transgenic Mice.Int J Mol Sci. 2021 Mar 13;22(6):2939. doi: 10.3390/ijms22062939. Int J Mol Sci. 2021. PMID: 33805843 Free PMC article.
-
A53T-α-synuclein overexpression in murine locus coeruleus induces Parkinson's disease-like pathology in neurons and glia.Acta Neuropathol Commun. 2018 May 10;6(1):39. doi: 10.1186/s40478-018-0541-1. Acta Neuropathol Commun. 2018. PMID: 29747690 Free PMC article.
-
Opposing effects of β-2 and β-1 adrenergic receptor signaling on neuroinflammation and dopaminergic neuron survival in α-synuclein-mediated neurotoxicity.J Neuroinflammation. 2023 Mar 2;20(1):56. doi: 10.1186/s12974-023-02748-3. J Neuroinflammation. 2023. PMID: 36864439 Free PMC article.
-
Targeting the norepinephrinergic system in Parkinson's disease and related disorders: The locus coeruleus story.Neurochem Int. 2017 Jan;102:22-32. doi: 10.1016/j.neuint.2016.11.009. Epub 2016 Nov 27. Neurochem Int. 2017. PMID: 27899296 Review.
-
Locus coeruleus.Cell Tissue Res. 2018 Jul;373(1):221-232. doi: 10.1007/s00441-017-2649-1. Epub 2017 Jul 7. Cell Tissue Res. 2018. PMID: 28687925 Review.
Cited by
-
Differential vulnerability of locus coeruleus and dorsal raphe neurons to chronic methamphetamine-induced degeneration.Front Cell Neurosci. 2022 Jul 22;16:949923. doi: 10.3389/fncel.2022.949923. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35936499 Free PMC article.
-
The noradrenergic subtype of Parkinson disease: from animal models to clinical practice.Nat Rev Neurol. 2023 Jun;19(6):333-345. doi: 10.1038/s41582-023-00802-5. Epub 2023 May 4. Nat Rev Neurol. 2023. PMID: 37142796 Review.
-
Cellular Models of Alpha-Synuclein Aggregation: What Have We Learned and Implications for Future Study.Biomedicines. 2022 Oct 20;10(10):2649. doi: 10.3390/biomedicines10102649. Biomedicines. 2022. PMID: 36289910 Free PMC article. Review.
-
Pathogenesis and treatment of perioperative hiccups: a narrative review.Ann Med. 2025 Dec;57(1):2474173. doi: 10.1080/07853890.2025.2474173. Epub 2025 Mar 8. Ann Med. 2025. PMID: 40055925 Free PMC article.
-
Pathogenesis of depression and the potential for traditional Chinese medicine treatment.Front Pharmacol. 2024 Jun 25;15:1407869. doi: 10.3389/fphar.2024.1407869. eCollection 2024. Front Pharmacol. 2024. PMID: 38983910 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

