Response-adapted anti-PD-1-based salvage therapy for Hodgkin lymphoma with nivolumab alone or in combination with ICE
- PMID: 35316328
- PMCID: PMC9227101
- DOI: 10.1182/blood.2022015423
Response-adapted anti-PD-1-based salvage therapy for Hodgkin lymphoma with nivolumab alone or in combination with ICE
Abstract
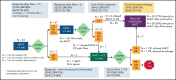
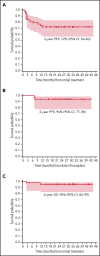
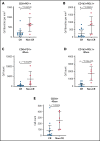
This phase 2 trial evaluated PET-adapted nivolumab alone or in combination with ifosfamide, carboplatin, and etoposide (NICE) as first salvage therapy and bridge to autologous hematopoietic cell transplantation (AHCT) in relapsed/refractory (RR) classical Hodgkin lymphoma (cHL). Patients with RR cHL received 240 mg nivolumab every 2 weeks for up to 6 cycles (C). Patients in complete response (CR) after C6 proceeded to AHCT, whereas patients with progressive disease at any point or not in CR after C6 received NICE for 2 cycles. The primary endpoint was CR rate per the 2014 Lugano classification at completion of protocol therapy. Forty-three patients were evaluable for toxicity; 42 were evaluable for response. Thirty-four patients received nivolumab alone, and 9 patients received nivolumab+NICE. No unexpected toxicities were observed after nivolumab or NICE. After nivolumab, the overall response rate (ORR) was 81%, and the CR rate was 71%. Among 9 patients who received NICE, all responded, with 8 (89%) achieving CR. At the end of protocol therapy, the ORR and CR rates were 93% and 91%. Thirty-three patients were bridged directly to AHCT, including 26 after Nivo alone. The 2-year progression-free survival (PFS) and overall survival in all treated patients (n = 43) were 72% and 95%, respectively. Among 33 patients who bridged directly to AHCT, the 2-year PFS was 94% (95% CI: 78-98). PET-adapted sequential salvage therapy with nivolumab/nivolumab+NICE was well tolerated and effective, resulting in a high CR rate and bridging most patients to AHCT without chemotherapy. This trial was registered at www.clinicaltrials.gov #NCT03016871.
© 2022 by The American Society of Hematology.
Figures


Comment in
-
Smart salvage treatment for Hodgkin lymphoma.Blood. 2022 Jun 23;139(25):3563-3564. doi: 10.1182/blood.2022016274. Blood. 2022. PMID: 35737409 No abstract available.
References
-
- Armitage JO. Early-stage Hodgkin’s lymphoma. N Engl J Med. 2010;363(7):653-662. - PubMed
-
- Engert A, Diehl V, Franklin J, et al. . Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin’s lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin Oncol. 2009;27(27):4548-4554. - PubMed
-
- Radford J, Illidge T, Counsell N, et al. . Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med. 2015;372(17):1598-1607. - PubMed

