Procedure providing SI-traceable results for the calibration of protein standards by sulfur determination and its application on tau
- PMID: 35316347
- PMCID: PMC9142460
- DOI: 10.1007/s00216-022-03974-z
Procedure providing SI-traceable results for the calibration of protein standards by sulfur determination and its application on tau
Abstract
Quantitative proteomics is a growing research area and one of the most important tools in the life sciences. Well-characterized and quantified protein standards are needed to achieve accurate and reliable results. However, only a limited number of sufficiently characterized protein standards are currently available. To fill this gap, a method for traceable protein quantification using sulfur isotope dilution inductively coupled plasma mass spectrometry (ICP-MS) was developed in this study. Gel filtration and membrane filtration were tested for the separation of non-protein-bound sulfur in the protein solution. Membrane filtration demonstrated a better performance due to the lower workload and the very low sulfur blanks of 11 ng, making it well suited for high-purity proteins such as NIST SRM 927, a bovine serum albumin (BSA). The method development was accomplished with NIST SRM 927e and a commercial avidin. The quantified mass fraction of NIST SRM 927e agreed very well with the certified value and showed similar uncertainties (3.6%) as established methods while requiring less sample preparation and no species-specific standards. Finally, the developed procedure was applied to the tau protein, which is a biomarker for a group of neurodegenerative diseases denoted "tauopathies" including, e.g., Alzheimer's disease and frontotemporal dementia. For the absolute quantification of tau in the brain of transgenic mice overexpressing human tau, a well-defined calibration standard was needed. Therefore, a pure tau solution was quantified, yielding a protein mass fraction of (0.328 ± 0.036) g/kg, which was confirmed by amino acid analysis.
Keywords: Inductively coupled plasma mass spectrometry; Isotope dilution; Quantitative protein analysis; SI traceability; Sulfur.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
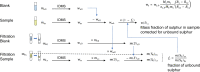

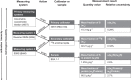
Similar articles
-
Certification of visinin-like protein-1 (VILIP-1) certified reference material by amino acid-based and sulfur-based liquid chromatography isotope dilution mass spectrometry.Anal Bioanal Chem. 2023 Jan;415(1):211-220. doi: 10.1007/s00216-022-04401-z. Epub 2022 Nov 7. Anal Bioanal Chem. 2023. PMID: 36342508
-
Development of a candidate reference measurement procedure by ID-LC-MS/MS for total tau protein measurement in cerebrospinal fluid (CSF).Clin Chem Lab Med. 2023 Feb 24;61(7):1235-1244. doi: 10.1515/cclm-2022-1250. Print 2023 Jun 27. Clin Chem Lab Med. 2023. PMID: 36815732
-
Determination of trace sulfur in biodiesel and diesel standard reference materials by isotope dilution sector field inductively coupled plasma mass spectrometry.Anal Chim Acta. 2014 Jan 2;806:91-6. doi: 10.1016/j.aca.2013.10.056. Epub 2013 Nov 14. Anal Chim Acta. 2014. PMID: 24331043
-
Quantitative aspects of inductively coupled plasma mass spectrometry.Philos Trans A Math Phys Eng Sci. 2016 Oct 28;374(2079):20150369. doi: 10.1098/rsta.2015.0369. Philos Trans A Math Phys Eng Sci. 2016. PMID: 27644971 Free PMC article. Review.
-
The role of sulfur and sulfur isotope dilution analysis in quantitative protein analysis.Anal Bioanal Chem. 2008 Jan;390(2):605-15. doi: 10.1007/s00216-007-1607-2. Epub 2007 Nov 6. Anal Bioanal Chem. 2008. PMID: 17985123 Review.
Cited by
-
Development of an Online Isotope Dilution CE/ICP-MS Method for the Quantification of Sulfur in Biological Compounds.Anal Chem. 2024 Feb 27;96(8):3276-3283. doi: 10.1021/acs.analchem.3c03553. Epub 2024 Jan 31. Anal Chem. 2024. PMID: 38294348 Free PMC article.
References
-
- Josephs RD, Martos G, Li M, Wu L, Melanson J, Quaglia M, et al. Establishment of measurement traceability for peptide and protein quantification through rigorous purity assessment–a review. Metrologia. 2019. 10.1088/1681-7575/ab27e5
-
- Bunk DM. Reference materials and reference measurement procedures: an overview from a national metrology institute. Clin. Biochem. Rev. 2007;28(4):131. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2282405/pdf/cbr28_4p131.pdf. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

