Real-World Treatment Patterns in Metastatic Castration-Resistant Prostate Cancer Across Europe (France, Germany, Italy, Spain, and the United Kingdom) and Japan
- PMID: 35316501
- PMCID: PMC9056448
- DOI: 10.1007/s12325-022-02073-w
Real-World Treatment Patterns in Metastatic Castration-Resistant Prostate Cancer Across Europe (France, Germany, Italy, Spain, and the United Kingdom) and Japan
Abstract
Introduction: Prostate cancer (PC) is the second most common cancer, and the fifth most common cause of cancer-related mortality among male patients, worldwide. In Europe and Japan, the incidence of PC in men in 2020 exceeded that of lung cancer. Although national and regional clinical guidelines for the treatment of metastatic castration-resistant prostate cancer (mCRPC) are available in Europe and Japan, a literature review did not identify a published comparison of differing guidelines, but identified a lack of studies reporting treatment patterns of approved mCRPC treatments in Europe and Japan in normal clinical practice. The objective of this real-world study was to compare national treatment guidelines and real-world treatment for mCRPC in Europe and Japan.
Methods: Physician-reported demographics, clinical characteristics, and treatment data of patients with mCRPC were drawn from the Adelphi Prostate Cancer Disease Specific Programme™, conducted in five European countries and Japan (2020) and analysed descriptively.
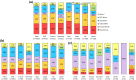
Results: All current treatment guidelines recommended the use of novel hormonal agents (NHA-abiraterone/enzalutamide) and chemotherapy (mainly docetaxel), with some intercountry differences, with NHA rechallenge accepted in Germany, Italy and Japan, but not in France, Spain or the United Kingdom. Overall, 271 physicians provided data for 1753 patients. At 1st-line (1L), the most common treatment was NHAs followed by (→) chemotherapy, in all countries. Chemotherapy was the most common 2nd-line (2L) treatment, except in Japan, where 2L NHA use was preferred, and Spain, where both were used equally. NHA → chemotherapy and chemotherapy → NHA were the first and second usual 1L → 2L sequence in most countries, except for France, where the second most common sequence was NHA → NHA, and Japan, with androgen deprivation therapy alone → NHA.
Conclusion: Real-world mCRPC treatment patterns largely reflected national guidelines. It is expected that guidelines and treatment patterns will change with the development of new treatment options.
Keywords: Chemotherapy; Docetaxel; Metastatic castration-resistant prostate cancer; Novel hormonal agents; Treatment guidelines; Treatment patterns.
© 2022. Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and Adelphi Real World, Bollington, UK [2022].
Figures
References
-
- Morgan C, et al. PCN17 Castration-resistant prostate cancer (CRPC): a UK epidemiology study. Value Health. 2010;13(3):A26. doi: 10.1016/S1098-3015(10)72108-2. - DOI
-
- Gravanis I, et al. The European medicines agency review of abiraterone for the treatment of metastatic castration-resistant prostate cancer in adult men after docetaxel chemotherapy and in chemotherapy-naive disease: summary of the scientific assessment of the committee for medicinal products for human use. Oncologist. 2013;18(9):1032–1042. doi: 10.1634/theoncologist.2013-0092. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources