A systematic review on mucocutaneous presentations after COVID-19 vaccination and expert recommendations about vaccination of important immune-mediated dermatologic disorders
- PMID: 35316551
- PMCID: PMC9111423
- DOI: 10.1111/dth.15461
A systematic review on mucocutaneous presentations after COVID-19 vaccination and expert recommendations about vaccination of important immune-mediated dermatologic disorders
Abstract
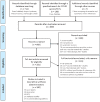
With dermatologic side effects being fairly prevalent following vaccination against COVID-19, and the multitude of studies aiming to report and analyze these adverse events, the need for an extensive investigation on previous studies seemed urgent, in order to provide a thorough body of information about these post-COVID-19 immunization mucocutaneous reactions. To achieve this goal, a comprehensive electronic search was performed through the international databases including Medline (PubMed), Scopus, Cochrane, Web of science, and Google scholar on July 12, 2021, and all articles regarding mucocutaneous manifestations and considerations after COVID-19 vaccine administration were retrieved using the following keywords: COVID-19 vaccine, dermatology considerations and mucocutaneous manifestations. A total of 917 records were retrieved and a final number of 180 articles were included in data extraction. Mild, moderate, severe and potentially life-threatening adverse events have been reported following immunization with COVID vaccines, through case reports, case series, observational studies, randomized clinical trials, and further recommendations and consensus position papers regarding vaccination. In this systematic review, we categorized these results in detail into five elaborate tables, making what we believe to be an extensively informative, unprecedented set of data on this topic. Based on our findings, in the viewpoint of the pros and cons of vaccination, mucocutaneous adverse events were mostly non-significant, self-limiting reactions, and for the more uncommon moderate to severe reactions, guidelines and consensus position papers could be of great importance to provide those at higher risks and those with specific worries of flare-ups or inefficient immunization, with sufficient recommendations to safely schedule their vaccine doses, or avoid vaccination if they have the discussed contra-indications.
Keywords: AstraZeneca; AstraZeneca/Oxford; Bharat; COVID-19 vaccine; Comirnaty; Janssen; Johnson & Johnson; Moderna; Pernio; Petechia; Pfizer; Pfizer-BioNTech; SARS-Cov-2; Sinopharm; Sinovac; Sputnik; Vaccine Adverse Event Reporting System; acute; adverse effect; adverse event; adverse event following immunization; allergy; angioedema; atopic dermatitis; collagen vascular disease; cutaneous; cyanosis; delayed; delayed-type hypersensitivity; dermatology; ecchymosis; edema; erythema multiforme; exanthematous rash; herpes; hidradenitis suppurativa; inflammatory bowel disease; injection site reaction; late; local site reaction; maculopapular rash; mastocytosis; morbilliform rash; mucocutaneous; mucosal; pemphigoid; pemphigus; pityriasis rosea; pruritus; psoriasis; purpura; remote site reaction; rheumatic disorders; side effect; urticaria; vaccine; zoster.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Similar articles
-
Efficacy and safety of COVID-19 vaccines.Cochrane Database Syst Rev. 2022 Dec 7;12(12):CD015477. doi: 10.1002/14651858.CD015477. Cochrane Database Syst Rev. 2022. PMID: 36473651 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Cutaneous adverse reactions of COVID-19 vaccines: A systematic review.Dermatol Ther. 2022 May;35(5):e15391. doi: 10.1111/dth.15391. Epub 2022 Mar 8. Dermatol Ther. 2022. PMID: 35194894 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
Herpesviruses reactivation following COVID-19 vaccination: a systematic review and meta-analysis.Eur J Med Res. 2023 Aug 10;28(1):278. doi: 10.1186/s40001-023-01238-9. Eur J Med Res. 2023. PMID: 37559096 Free PMC article.
Cited by
-
Development of a Scoring Method Based on a Chest CT Scan to Determine the Outcomes of COVID-19 Patients.Cureus. 2023 Oct 19;15(10):e47354. doi: 10.7759/cureus.47354. eCollection 2023 Oct. Cureus. 2023. PMID: 38022268 Free PMC article.
-
Allergic Reactions to COVID-19 Vaccination in High-Risk Allergic Patients: The Experience of Trieste University Hospital (North-Eastern Italy).Vaccines (Basel). 2022 Sep 27;10(10):1616. doi: 10.3390/vaccines10101616. Vaccines (Basel). 2022. PMID: 36298481 Free PMC article.
-
Severe aggravation and possible triggering of pemphigus vulgaris following COVID-19 vaccination: report of two cases.An Bras Dermatol. 2024 Sep-Oct;99(5):758-762. doi: 10.1016/j.abd.2023.04.012. Epub 2024 Jun 3. An Bras Dermatol. 2024. PMID: 38834398 Free PMC article. No abstract available.
-
Could Vaccination against COVID-19 Trigger Immune-Mediated Inflammatory Diseases?J Clin Med. 2024 Aug 7;13(16):4617. doi: 10.3390/jcm13164617. J Clin Med. 2024. PMID: 39200759 Free PMC article.
-
Pathophysiology of oral lesions subsequent to SARS-CoV-2 vaccination: A systematic review.J Oral Maxillofac Pathol. 2024 Jul-Sep;28(3):443-454. doi: 10.4103/jomfp.jomfp_511_23. Epub 2024 Oct 15. J Oral Maxillofac Pathol. 2024. PMID: 39670118 Free PMC article. Review.
References
-
- Sahin U, Muik A, Vogler I, et al. BNT162b2 vaccine induces neutralizing antibodies and poly‐specific T cells in humans. Nature. 2021;595(7868):572‐577. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous