Pectoralis major muscle atrophy is associated with mitochondrial energy wasting in cachectic patients with gastrointestinal cancer
- PMID: 35316572
- PMCID: PMC9178397
- DOI: 10.1002/jcsm.12984
Pectoralis major muscle atrophy is associated with mitochondrial energy wasting in cachectic patients with gastrointestinal cancer
Abstract
Background: Cancer cachexia is a multifactorial syndrome characterized by involuntary and pathological weight loss, mainly due to skeletal muscle wasting, resulting in a decrease in patients' quality of life, response to cancer treatments, and survival. Our objective was to investigate skeletal muscle alterations in cachectic cancer patients.
Methods: This is a prospective study of patients managed for pancreatic or colorectal cancer with an indication for systemic chemotherapy (METERMUCADIG - NCT02573974). One lumbar CT image was used to determine body composition. Patients were divided into three groups [8 noncachectic (NC), 18 with mild cachexia (MC), and 19 with severe cachexia (SC)] based on the severity of weight loss and muscle mass. For each patient, a pectoralis major muscle biopsy was collected at the time of implantable chamber placement. We used high-resolution oxygraphy to measure mitochondrial muscle oxygen consumption on permeabilized muscle fibres. We also performed optical and electron microscopy analyses, as well as gene and protein expression analyses.
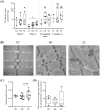
Results: Forty-five patients were included. Patients were 67% male, aged 67 years (interquartile range, 59-77). Twenty-three (51%) and 22 (49%) patients were managed for pancreatic and colorectal cancer, respectively. Our results show a positive correlation between median myofibres area and skeletal muscle index (P = 0.0007). Cancer cachexia was associated with a decrease in MAFbx protein expression (P < 0.01), a marker of proteolysis through the ubiquitin-proteasome pathway. Mitochondrial oxygen consumption related to energy wasting was significantly increased (SC vs. NC, P = 0.028) and mitochondrial area tended to increase (SC vs. MC, P = 0.056) in SC patients. On the contrary, mitochondria content and networks remain unaltered in cachectic cancer patients. Finally, our results show no dysfunction in lipid storage and endoplasmic reticulum homeostasis.
Conclusions: This clinical protocol brings unique data that provide new insight to mechanisms underlying muscle wasting in cancer cachexia. We report for the first time an increase in mitochondrial energy wasting in the skeletal muscle of severe cachectic cancer patients. Additional clinical studies are essential to further the exploring and understanding of these alterations.
Keywords: Cancer cachexia; Clinical study; Mitochondrial bioenergetics; Myosteatosis; Pectoralis major; Proteolysis.
© 2022 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
The authors declare that they have no conflict of interest in connection with this article.
Figures




References
-
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. - PubMed
-
- Bachmann J, Heiligensetzer M, Krakowski‐Roosen H, Büchler MW, Friess H, Martignoni ME. Cachexia worsens prognosis in patients with resectable pancreatic cancer. J Gastrointest Surg 2008;12:1193–1201. - PubMed
-
- Fearon KC, Voss AC, Hustead DS, Cancer Cachexia Study Group . Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr 2006;83:1345–1350. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

