Fluorescence Imaging of Neural Activity, Neurochemical Dynamics, and Drug-Specific Receptor Conformation with Genetically Encoded Sensors
- PMID: 35316611
- PMCID: PMC9940643
- DOI: 10.1146/annurev-neuro-110520-031137
Fluorescence Imaging of Neural Activity, Neurochemical Dynamics, and Drug-Specific Receptor Conformation with Genetically Encoded Sensors
Abstract
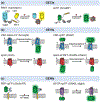
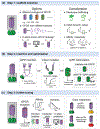
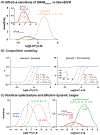
Recent advances in fluorescence imaging permit large-scale recording of neural activity and dynamics of neurochemical release with unprecedented resolution in behaving animals. Calcium imaging with highly optimized genetically encoded indicators provides a mesoscopic view of neural activity from genetically defined populations at cellular and subcellular resolutions. Rigorously improved voltage sensors and microscopy allow for robust spike imaging of populational neurons in various brain regions. In addition, recent protein engineering efforts in the past few years have led to the development of sensors for neurotransmitters and neuromodulators. Here, we discuss the development and applications of these genetically encoded fluorescent indicators in reporting neural activity in response to various behaviors in different biological systems as well as in drug discovery. We also report a simple model to guide sensor selection and optimization.
Keywords: biosensors; drug discovery; fluorescence imaging; genetically encoded indicators; neurocircuit imaging; neuromodulation.
Figures

References
-
- Abdelfattah AS, Kawashima T, Singh A, Novak O, Liu H, et al. 2019. Bright and photostable chemigenetic indicators for extended in vivo voltage imaging. Science 365: 699–704 - PubMed
-
- Andreoni A, Davis CMO, Tian L. 2019. Measuring brain chemistry using genetically encoded fluorescent sensors. Current Opinion in Biomedical Engineering 12: 59–67
-
- Borden PM, Zhang P, Shivange AV, Marvin JS, Cichon J, et al. 2020. A fast genetically encoded fluorescent sensor for faithful in vivo acetylcholine detection in mice, fish, worms and flies. bioRxiv: 2020.02.07.939504
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

