The central moTOR of metabolism
- PMID: 35316619
- PMCID: PMC9004513
- DOI: 10.1016/j.devcel.2022.02.024
The central moTOR of metabolism
Abstract
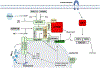
The protein kinase mechanistic target of rapamycin (mTOR) functions as a central regulator of metabolism, integrating diverse nutritional and hormonal cues to control anabolic processes, organismal physiology, and even aging. This review discusses the current state of knowledge regarding the regulation of mTOR signaling and the metabolic regulation of the four macromolecular building blocks of the cell: carbohydrate, nucleic acid, lipid, and protein by mTOR. We review the role of mTOR in the control of organismal physiology and aging through its action in key tissues and discuss the potential for clinical translation of mTOR inhibition for the treatment and prevention of diseases of aging.
Keywords: amino acids; lipids; mTOR; mTORC1; mTORC2; metabolism; protein; rapamycin.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests D.W.L. has received funding from and is a scientific advisory board member of Aeovian Pharmaceuticals, which seeks to develop novel, selective mTOR inhibitors for the treatment of various diseases.
Figures


References
-
- Arriola Apelo SI, and Lamming DW (2016). mTORC2 Puts Its Shoulder to Krebs’ Wheel. Mol Cell 63, 723–725. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

