Fungal sensing enhances neutrophil metabolic fitness by regulating antifungal Glut1 activity
- PMID: 35316647
- PMCID: PMC9026661
- DOI: 10.1016/j.chom.2022.02.017
Fungal sensing enhances neutrophil metabolic fitness by regulating antifungal Glut1 activity
Abstract
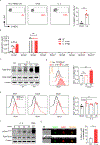
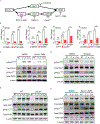
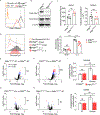
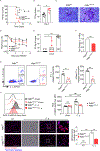
Combating fungal pathogens poses metabolic challenges for neutrophils, key innate cells in anti-Candida albicans immunity, yet how host-pathogen interactions cause remodeling of the neutrophil metabolism is unclear. We show that neutrophils mediate renal immunity to disseminated candidiasis by upregulating glucose uptake via selective expression of glucose transporter 1 (Glut1). Mechanistically, dectin-1-mediated recognition of β-glucan leads to activation of PKCδ, which triggers phosphorylation, localization, and early glucose transport by a pool of pre-formed Glut1 in neutrophils. These events are followed by increased Glut1 gene transcription, leading to more sustained Glut1 accumulation, which is also dependent on the β-glucan/dectin-1/CARD9 axis. Card9-deficient neutrophils show diminished glucose incorporation in candidiasis. Neutrophil-specific Glut1-ablated mice exhibit increased mortality in candidiasis caused by compromised neutrophil phagocytosis, reactive oxygen species (ROS), and neutrophil extracellular trap (NET) formation. In human neutrophils, β-glucan triggers metabolic remodeling and enhances candidacidal function. Our data show that the host-pathogen interface increases glycolytic activity in neutrophils by regulating Glut1 expression, localization, and function.
Keywords: Candida albicans; fungus; glucose; glucose transporter 1; immunometabolism; kidney; neutrophils.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests
Figures


Comment in
-
Neutrophils flash their GLUTs to beat back detestable fungi.Cell Host Microbe. 2022 Apr 13;30(4):415-417. doi: 10.1016/j.chom.2022.03.027. Cell Host Microbe. 2022. PMID: 35421332
Similar articles
-
Neutrophil Attack Triggers Extracellular Trap-Dependent Candida Cell Wall Remodeling and Altered Immune Recognition.PLoS Pathog. 2016 May 25;12(5):e1005644. doi: 10.1371/journal.ppat.1005644. eCollection 2016 May. PLoS Pathog. 2016. PMID: 27223610 Free PMC article.
-
CARD9+ microglia promote antifungal immunity via IL-1β- and CXCL1-mediated neutrophil recruitment.Nat Immunol. 2019 May;20(5):559-570. doi: 10.1038/s41590-019-0377-2. Epub 2019 Apr 17. Nat Immunol. 2019. PMID: 30996332 Free PMC article.
-
Delinking CARD9 and IL-17: CARD9 Protects against Candida tropicalis Infection through a TNF-α-Dependent, IL-17-Independent Mechanism.J Immunol. 2015 Oct 15;195(8):3781-92. doi: 10.4049/jimmunol.1500870. Epub 2015 Sep 2. J Immunol. 2015. PMID: 26336150 Free PMC article.
-
CARD9 Syk-dependent and Raf-1 Syk-independent signaling pathways in target recognition of Candida albicans by Dectin-1.Eur J Clin Microbiol Infect Dis. 2011 Mar;30(3):303-5. doi: 10.1007/s10096-010-1103-z. Epub 2010 Nov 25. Eur J Clin Microbiol Infect Dis. 2011. PMID: 21108038 Review.
-
Neutrophil Extracellular Traps in Candida albicans Infection.Front Immunol. 2022 Jun 16;13:913028. doi: 10.3389/fimmu.2022.913028. eCollection 2022. Front Immunol. 2022. PMID: 35784323 Free PMC article. Review.
Cited by
-
The pathobiology of human fungal infections.Nat Rev Microbiol. 2024 Nov;22(11):687-704. doi: 10.1038/s41579-024-01062-w. Epub 2024 Jun 25. Nat Rev Microbiol. 2024. PMID: 38918447 Review.
-
Immune responses to human fungal pathogens and therapeutic prospects.Nat Rev Immunol. 2023 Jul;23(7):433-452. doi: 10.1038/s41577-022-00826-w. Epub 2023 Jan 4. Nat Rev Immunol. 2023. PMID: 36600071 Free PMC article. Review.
-
Mild Intermittent Cold Stimulation Affects Cardiac Substance Metabolism via the Neuroendocrine Pathway in Broilers.Animals (Basel). 2023 Nov 19;13(22):3577. doi: 10.3390/ani13223577. Animals (Basel). 2023. PMID: 38003194 Free PMC article.
-
The Interplay between Candida albicans, Vaginal Mucosa, Host Immunity and Resident Microbiota in Health and Disease: An Overview and Future Perspectives.Microorganisms. 2023 May 5;11(5):1211. doi: 10.3390/microorganisms11051211. Microorganisms. 2023. PMID: 37317186 Free PMC article. Review.
-
Metabolic homeostasis in fungal infections from the perspective of pathogens, immune cells, and whole-body systems.Microbiol Mol Biol Rev. 2024 Sep 26;88(3):e0017122. doi: 10.1128/mmbr.00171-22. Epub 2024 Sep 4. Microbiol Mol Biol Rev. 2024. PMID: 39230301 Review.
References
-
- Ardati KO, Bajakian KM, and Tabbara KS (1997). Effect of glucose-6-phosphate dehydrogenase deficiency on neutrophil function. Acta Haematol 97, 211–215. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

