Associations between hemostatic markers and mortality in COVID-19 - Compounding effects of D-dimer, antithrombin and PAP complex
- PMID: 35316719
- PMCID: PMC8930184
- DOI: 10.1016/j.thromres.2022.03.013
Associations between hemostatic markers and mortality in COVID-19 - Compounding effects of D-dimer, antithrombin and PAP complex
Abstract
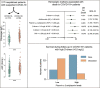
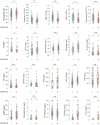
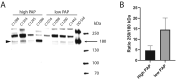
In this single-center cohort study, we applied a panel of laboratory markers to characterize hemostatic function in 217 consecutive patients that underwent testing for COVID-19 as they were admitted to Linköping University Hospital between April and June 2020. In the 96 patients that tested positive for SARS-CoV-2 (COVID-19+), the cumulative incidences of death and venous thromboembolism were 24.0% and 19.8% as compared to 12.4% (p = 0.031) and 11.6% (p = 0.13) in the 121 patients that tested negative (COVID-19-). In COVID-19+ patients, we found pronounced increases in plasma levels of von Willebrand factor (vWF) and fibrinogen. Excess mortality was observed in COVID-19+ patients with the following aberrations in hemostatic markers: high D-dimer, low antithrombin or low plasmin-antiplasmin complex (PAP) formation, with Odds Ratios (OR) for death of 4.7 (95% confidence interval (CI95) 1.7-12.9; p = 0.003) for D-dimer >0.5 mg/L, 5.9 (CI95 1.8-19.7; p = 0.004) for antithrombin (AT) ˂0.85 kIU/l and 4.9 (CI95 1.3-18.3; p = 0.019) for PAP < 1000 μg/L. Compounding increases in mortality was observed in COVID-19+ patients with combined defects in markers of fibrinolysis and coagulation, with ORs for death of 15.7 (CI95 4.3-57; p < 0.001) for patients with PAP <1000 μg/L and D-dimer >0.5 mg/L and 15.5 (CI95 2.8-87, p = 0.002) for patients with PAP <1000 μg/L and AT ˂0.85 kIU/L. We observed an elevated fraction of incompletely degraded D-dimer fragments in COVID-19+ patients with low PAP, indicating impaired fibrinolytic breakdown of cross-linked fibrin.
Keywords: Antithrombin; COVID-19; Fibrinolysis; Plasmin-antiplasmin complex.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

