Population pharmacokinetics of mobocertinib in healthy volunteers and patients with non-small cell lung cancer
- PMID: 35316867
- PMCID: PMC9197538
- DOI: 10.1002/psp4.12785
Population pharmacokinetics of mobocertinib in healthy volunteers and patients with non-small cell lung cancer
Abstract
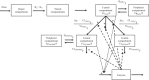
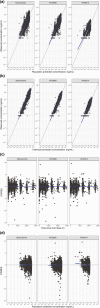
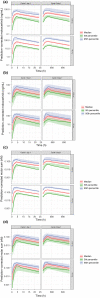
Mobocertinib is an oral tyrosine kinase inhibitor approved for treatment of patients with locally advanced or metastatic non-small cell lung cancer (mNSCLC) with epidermal growth factor receptor gene (EGFR) exon 20 insertion mutations whose disease has progressed on or after platinum-based chemotherapy. This population pharmacokinetic (PK) analysis describes the PK of mobocertinib and its active metabolites, AP32960, and AP32914, using data from two phase I studies in healthy volunteers (n = 110) and two phase I/II studies in patients with mNSCLC (n = 317), including the pivotal phase I/II study. The plasma PK of mobocertinib, AP32960, and AP32914 were well-characterized by a joint semimechanistic model that included two compartments for mobocertinib with absorption via three transit compartments, two compartments for AP32960, and one compartment for AP32914. The observed time-dependency in PK was described by an enzyme compartment with drug and metabolite concentration-dependent stimulation of enzyme production, resulting in the enzyme increasing the apparent clearance of mobocertinib, AP32960, and AP32914. Effects of healthy volunteer status (vs. patients with mNSCLC) on apparent oral clearance of all three moieties and on apparent central volume of distribution for mobocertinib were included as structural covariates in the final model. No clinically meaningful differences in mobocertinib PK were observed based on age (18-86 years), race, sex, body weight (37.3-132 kg), mild-to-moderate renal impairment (estimated glomerular filtration rate 30-89 ml/min/1.73 m2 by modification of diet in renal disease equation), or mild-to-moderate hepatic impairment, suggesting that no dose adjustment is required based on these covariates in patients with mNSCLC.
© 2022 Takeda Pharmaceuticals. CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Neeraj Gupta is employed by Takeda; Philippe B. Pierrillas is employed by Certara and is a consultant for Takeda; Michael J. Hanley is employed by Takeda; Steven Zhang is employed by Takeda; Paul M. Diderichsen is employed by Certara and is a consultant for Takeda.
Figures

References
-
- Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non‐small‐cell lung cancer: preclinical data and clinical implications. Lancet Oncol. 2012;13:e23‐e31. - PubMed
-
- O'Kane GM, Bradbury PA, Feld R, et al. Uncommon EGFR mutations in advanced non‐small cell lung cancer. Lung Cancer. 2017;109:137‐144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

