Analytical Modeling of Flowrate and Its Maxima in Electrochemical Bioelectronics with Drug Delivery Capabilities
- PMID: 35316891
- PMCID: PMC8917966
- DOI: 10.34133/2022/9805932
Analytical Modeling of Flowrate and Its Maxima in Electrochemical Bioelectronics with Drug Delivery Capabilities
Abstract
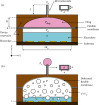
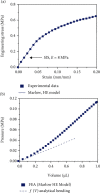
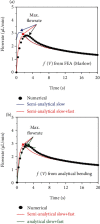
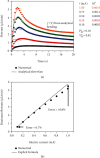
Flowrate control in flexible bioelectronics with targeted drug delivery capabilities is essential to ensure timely and safe delivery. For neuroscience and pharmacogenetics studies in small animals, these flexible bioelectronic systems can be tailored to deliver small drug volumes on a controlled fashion without damaging surrounding tissues from stresses induced by excessively high flowrates. The drug delivery process is realized by an electrochemical reaction that pressurizes the internal bioelectronic chambers to deform a flexible polymer membrane that pumps the drug through a network of microchannels implanted in the small animal. The flowrate temporal profile and global maximum are governed and can be modeled by the ideal gas law. Here, we obtain an analytical solution that groups the relevant mechanical, fluidic, environmental, and electrochemical terms involved in the drug delivery process into a set of three nondimensional parameters. The unique combinations of these three nondimensional parameters (related to the initial pressure, initial gas volume, and microfluidic resistance) can be used to model the flowrate and scale up the flexible bioelectronic design for experiments in medium and large animal models. The analytical solution is divided into (1) a fast variable that controls the maximum flowrate and (2) a slow variable that models the temporal profile. Together, the two variables detail the complete drug delivery process and control using the three nondimensional parameters. Comparison of the analytical model with alternative numerical models shows excellent agreement and validates the analytic modeling approach. These findings serve as a theoretical framework to design and optimize future flexible bioelectronic systems used in biomedical research, or related medical fields, and analytically control the flowrate and its global maximum for successful drug delivery.
Copyright © 2022 Raudel Avila et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Modeling programmable drug delivery in bioelectronics with electrochemical actuation.Proc Natl Acad Sci U S A. 2021 Mar 16;118(11):e2026405118. doi: 10.1073/pnas.2026405118. Proc Natl Acad Sci U S A. 2021. PMID: 33836613 Free PMC article.
-
Wearable Bioelectronics: Enzyme-Based Body-Worn Electronic Devices.Acc Chem Res. 2018 Nov 20;51(11):2820-2828. doi: 10.1021/acs.accounts.8b00451. Epub 2018 Nov 6. Acc Chem Res. 2018. PMID: 30398344 Free PMC article.
-
Organic bioelectronics in medicine.J Intern Med. 2017 Jul;282(1):24-36. doi: 10.1111/joim.12595. Epub 2017 Feb 9. J Intern Med. 2017. PMID: 28181720 Review.
-
Programmable intratumoral drug delivery to breast cancer using wireless bioelectronic device with electrochemical actuation.Expert Opin Drug Deliv. 2024 Mar;21(3):495-511. doi: 10.1080/17425247.2024.2323211. Epub 2024 Feb 28. Expert Opin Drug Deliv. 2024. PMID: 38396366
-
Wearable and Implantable Soft Bioelectronics Using Two-Dimensional Materials.Acc Chem Res. 2019 Jan 15;52(1):73-81. doi: 10.1021/acs.accounts.8b00491. Epub 2018 Dec 26. Acc Chem Res. 2019. PMID: 30586292 Review.
Cited by
-
An integrated microfluidic and fluorescence platform for probing in vivo neuropharmacology.Neuron. 2025 May 21;113(10):1491-1506.e6. doi: 10.1016/j.neuron.2025.03.017. Epub 2025 Apr 10. Neuron. 2025. PMID: 40215966
References
-
- Bußmann A. B., Grünerbel L. M., Durasiewicz C. P., Thalhofer T. A., Wille A., Richter M. Microdosing for drug delivery application—a review. Sensors and Actuators, A: Physical . 2021;330:p. 112820. doi: 10.1016/j.sna.2021.112820. - DOI
-
- Kumar A., Pillai J. Implantable drug delivery systems: an overview. Nanostructures for the Engineering of Cells, Tissues and Organs . 2018:473–511. doi: 10.1016/B978-0-12-813665-2.00013-2. - DOI
LinkOut - more resources
Full Text Sources

