Treatment adherence and effect of concurrent statin intensity on the efficacy and safety of alirocumab in a real-life setting: results from ODYSSEY APPRISE
- PMID: 35316922
- PMCID: PMC8924821
- DOI: 10.5114/aoms/143476
Treatment adherence and effect of concurrent statin intensity on the efficacy and safety of alirocumab in a real-life setting: results from ODYSSEY APPRISE
Abstract
Introduction: The phase IIIb open-label ODYSSEY APPRISE study prospectively assessed the safety and efficacy of alirocumab (a proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitor) in a real-life setting in high cardiovascular risk patients with heterozygous familial hypercholesterolemia or low-density lipoprotein cholesterol (LDL-C) not at goal despite maximally tolerated dose statins ± other lipid-lowering therapies (NCT02476006). This post-hoc analysis assessed patient adherence to statins and alirocumab, plus alirocumab efficacy and safety, according to concomitant statin intensity and prior ezetimibe usage.
Material and methods: Patients received alirocumab 75 or 150 mg (dose adjustment based on physician's judgment) every 2 weeks (for ≥ 3 to ≤ 30 months).
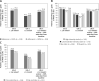
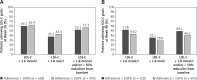
Results: Of 994 enrolled and treated patients, 58.4% received concomitant high-intensity statins, 18.2% received moderate/low-intensity statins, and 23.4% received no statin; 55.9% received prior ezetimibe. Mean alirocumab adherence (percent adherence defined as injections received/theoretical injections × 100) was 96.6% over 72.4 weeks' mean treatment duration. Mean LDL-C reduction from baseline at Week 12 was similar between statin intensity subgroups (53.6-55.7%). More patients achieved LDL-C < 1.8 mmol/l and/or ≥ 50% reduction from baseline in the ≥ 100% versus < 100% adherent to alirocumab subgroup; high-intensity and low/moderate-intensity subgroups versus no statin subgroup; and prior ezetimibe versus no prior ezetimibe subgroup. Treatment-emergent adverse events occurred in 65.2-75.1% and 68.0-76.3% of patients across statin and ezetimibe subgroups, respectively.
Conclusions: In a real-life setting, patient adherence to alirocumab was high. Alirocumab provided clinically significant reductions in LDL-C, with most patients achieving LDL-C treatment targets across background statin therapy and prior ezetimibe therapy subgroups.
Keywords: PCSK9; cholesterol; low-density lipoprotein; treatment adherence.
Copyright: © 2021 Termedia & Banach.
Figures

Similar articles
-
ODYSSEY EAST: Alirocumab efficacy and safety vs ezetimibe in high cardiovascular risk patients with hypercholesterolemia and on maximally tolerated statin in China, India, and Thailand.J Clin Lipidol. 2020 Jan-Feb;14(1):98-108.e8. doi: 10.1016/j.jacl.2019.10.015. Epub 2019 Nov 18. J Clin Lipidol. 2020. PMID: 31882376 Clinical Trial.
-
Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial.Eur Heart J. 2015 May 14;36(19):1186-94. doi: 10.1093/eurheartj/ehv028. Epub 2015 Feb 16. Eur Heart J. 2015. PMID: 25687353 Free PMC article. Clinical Trial.
-
[Efficacy and safety of alirocumab versus ezetimibe in high cardiovascular risk Chinese patients with hyperlipidemia: ODYSSEY EAST Study-Chinese sub-population analysis].Zhonghua Xin Xue Guan Bing Za Zhi. 2020 Jul 24;48(7):593-599. doi: 10.3760/cma.j.cn112148-20191216-00755. Zhonghua Xin Xue Guan Bing Za Zhi. 2020. PMID: 32842270 Clinical Trial. Chinese.
-
Alirocumab: A Review in Hypercholesterolemia.Am J Cardiovasc Drugs. 2016 Apr;16(2):141-52. doi: 10.1007/s40256-016-0166-3. Am J Cardiovasc Drugs. 2016. PMID: 26935836 Review.
-
Alirocumab for the treatment of hypercholesterolemia.Expert Opin Biol Ther. 2017 May;17(5):633-643. doi: 10.1080/14712598.2017.1305354. Epub 2017 Mar 20. Expert Opin Biol Ther. 2017. PMID: 28277798 Review.
Cited by
-
Regional differences in physicians' behavior and factors influencing the intensity of PCSK9 inhibitor therapy with alirocumab: a subanalysis of the ODYSSEY APPRISE study.Front Cardiovasc Med. 2023 Jun 19;10:1206551. doi: 10.3389/fcvm.2023.1206551. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37404744 Free PMC article.
-
Evaluation of miRNA-27a/b expression in patients with familial hypercholesterolemia.Arch Med Sci. 2022 May 29;20(4):1314-1320. doi: 10.5114/aoms/150500. eCollection 2024. Arch Med Sci. 2022. PMID: 39439685 Free PMC article.
-
Evinacumab, an ANGPTL3 Inhibitor, in the Treatment of Dyslipidemia.J Clin Med. 2022 Dec 25;12(1):168. doi: 10.3390/jcm12010168. J Clin Med. 2022. PMID: 36614969 Free PMC article. Review.
-
LDL lowering effect of PCSK9 inhibition is reduced in women.Eur Heart J Cardiovasc Pharmacother. 2023 Jun 2;9(4):337-342. doi: 10.1093/ehjcvp/pvad009. Eur Heart J Cardiovasc Pharmacother. 2023. PMID: 36722156 Free PMC article.
-
Position paper of the Polish Expert Group on the use of pitavastatin in the treatment of lipid disorders in Poland endorsed by the Polish Lipid Association.Arch Med Sci. 2023 Nov 26;20(1):28-42. doi: 10.5114/aoms/175879. eCollection 2024. Arch Med Sci. 2023. PMID: 38414478 Free PMC article.
References
-
- Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–88. - PubMed
-
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168–209. - PubMed
-
- Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics – 2019 update: A report from the American Heart Association. Circulation. 2019;139:e56–528. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
