First-line cisplatin, docetaxel, and cetuximab for patients with recurrent or metastatic head and neck cancer: A multicenter cohort study
- PMID: 35316930
- PMCID: PMC8894270
- DOI: 10.5306/wjco.v13.i2.147
First-line cisplatin, docetaxel, and cetuximab for patients with recurrent or metastatic head and neck cancer: A multicenter cohort study
Abstract
Background: The targeted therapy cetuximab [directed at the epidermal growth factor receptor (EGFR)] in combination with 5-fluorouracil and platinum-based chemotherapy (the EXTREME regimen) has shown substantial efficacy for patients with recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN). Thus, this scheme has been established as the preferred first-line option for these patients. However, more recently, a new strategy combining platinum, taxanes, and cetuximab (the TPEx regimen) has demonstrated similar efficacy with a more favorable toxicity profile in clinical trials.
Aim: To evaluate the safety and efficacy of the TPEx scheme as first-line therapy in advanced SCCHN in a multicenter cohort study.
Methods: This retrospective multicenter cohort study included patients with histologically confirmed recurrent or metastatic SCCHN treated with first-line TPEx at five medical centers in Argentina between January 1, 2017 and April 31, 2020. Chemotherapy consisted of four cycles of docetaxel, cisplatin, and cetuximab followed by cetuximab maintenance therapy. Clinical outcomes and toxicity profiles were collected from medical charts. Treatment response was assessed by the investigator in accordance with Response Evaluation Criteria in Solid Tumors (version 1.1). Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0).
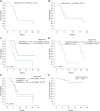
Results: Twenty-four patients were included. The median age at diagnosis was 58 years (range: 36-77 years). The majority of patients (83.3%) received at least four chemotherapy cycles in the initial phase. In the included group, the overall response rate was 62.5%, and 3 patients achieved a complete response (12.5%). The median time to response was 2.4 mo [95% confidence interval (CI): 1.3-3.5]. With a median follow-up of 12.7 mo (95%CI: 8.8-16.6), the median progression-free survival (PFS) was 6.9 mo (95%CI: 6.5-7.3), and the overall survival rate at 12 mo was 82.4%. Patients with documented tumor response showed a better PFS than those with disease stabilization or progression [8.5 mo (95%CI: 5.5-11.5) and 4.5 mo (95%CI: 2.5-6.6), respectively; P = 0.042]. Regarding the safety analysis, two-thirds of patients reported at least one treatment-related adverse event, and 25% presented grade 3 toxicities. Of note, no patient experienced grade 4 adverse events.
Conclusion: TPEx was an adequately tolerated regimen in our population, with low incidence of grade 3-4 adverse events. The median PFS were consistent with those in recent reports of clinical trials evaluating this treatment combination. This regimen may be considered an attractive therapeutic strategy due to its simplified administration, decreased total number of chemotherapy cycles, and treatment tolerability.
Keywords: Cetuximab; Cisplatin; Docetaxel; First-line; Recurrent and/or metastatic head and neck cancer; TPEx schema.
©The Author(s) 2022. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors have no conflicts of interest related to the manuscript.
Figures
References
-
- Mehanna H, Paleri V, West CM, Nutting C. Head and neck cancer--Part 1: Epidemiology, presentation, and prevention. BMJ. 2010;341:c4684. - PubMed
-
- Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N Engl J Med. 1993;328:184–194. - PubMed
-
- Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D, Peyrade F, Benasso M, Vynnychenko I, De Raucourt D, Bokemeyer C, Schueler A, Amellal N, Hitt R. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. - PubMed
-
- Mendenhall WM, Werning JW, Pfister DG. Cancer of the Head and Neck. In: Devita, Hellman, and Rosenberg’s cancer: principles & practice of oncology. 10th edition. Wolters Kluwer Health.
-
- Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Geese WJ, Kopit J, Shaw JW, Gillison ML. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375:1856–1867. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

