Strategies and Considerations for Improving Recombinant Antibody Production and Quality in Chinese Hamster Ovary Cells
- PMID: 35316944
- PMCID: PMC8934426
- DOI: 10.3389/fbioe.2022.856049
Strategies and Considerations for Improving Recombinant Antibody Production and Quality in Chinese Hamster Ovary Cells
Abstract
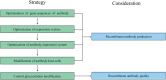
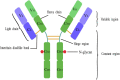
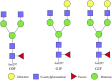
Recombinant antibodies are rapidly developing therapeutic agents; approximately 40 novel antibody molecules enter clinical trials each year, most of which are produced from Chinese hamster ovary (CHO) cells. However, one of the major bottlenecks restricting the development of antibody drugs is how to perform high-level expression and production of recombinant antibodies. The high-efficiency expression and quality of recombinant antibodies in CHO cells is determined by multiple factors. This review provides a comprehensive overview of several state-of-the-art approaches, such as optimization of gene sequence of antibody, construction and optimization of high-efficiency expression vector, using antibody expression system, transformation of host cell lines, and glycosylation modification. Finally, the authors discuss the potential of large-scale production of recombinant antibodies and development of culture processes for biopharmaceutical manufacturing in the future.
Keywords: Chinese hamster ovary cells; expression vector; genetic engineering; glycosylation; recombinant antibody.
Copyright © 2022 Zhang, Shan, Liang, Du and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- An Z. Q. (2009). Therapeutic Monoclonal Antibodies: From Bench to Clinic. New Jersey: John Wiley & Sons, 73–75.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

