Relationship between clinical remission of perianal fistulas in Crohn's disease and serum adalimumab concentrations: A multi-center cross-sectional study
- PMID: 35317057
- PMCID: PMC8908286
- DOI: 10.3748/wjg.v28.i9.961
Relationship between clinical remission of perianal fistulas in Crohn's disease and serum adalimumab concentrations: A multi-center cross-sectional study
Abstract
Background: Crohn's disease (CD) is complicated by perianal fistulas in approximately 20% of patients. Achieving permanent fistula closure remains a challenge for physicians. An association between serum anti-tumor necrosis factor-α concentrations and clinical outcomes in patients with CD has been demonstrated; however, little information is available on serum adalimumab (ADA) concentrations and remission of perianal fistulas in such patients.
Aim: To study the relationship between serum ADA concentrations and clinical remission of CD-associated perianal fistulas.
Methods: This cross-sectional study of patients with CD-associated perianal fistulas treated with ADA was performed at four French hospitals between December 2013 and March 2018. At the time of each serum ADA concentration measurement, we collected information about the patients and their fistulas. The primary study endpoint was clinical remission of fistulas defined as the absence of drainage (in accordance with Present's criteria), with a PDAI ≤ 4, absence of a seton and assessment of the overall evaluation as favorable by the proctologist at the relevant center. We also assessed fistula healing [defined as being in clinical and radiological (magnetic resonance imaging, MRI) remission] and adverse events.
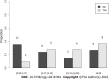
Results: The study cohort comprised 34 patients who underwent 56 evaluations (patients had between one and four evaluations). Fifteen patients had clinical remissions (44%), four of whom had healed fistulas on MRI. Serum ADA concentrations were significantly higher at evaluations in which clinical remission was identified than at evaluations in which it was not [14 (10-16) vs 10 (2-15) μg/mL, P = 0.01]. Serum ADA concentrations were comparable at the times of evaluation of patients with and without healed fistulas [11 (7-14) vs 10 (4-16) μg/mL, P = 0.69]. The adverse event rate did not differ between different serum ADA concentrations.
Conclusion: We found a significant association between high serum ADA concentrations and clinical remission of CD-associated perianal fistulas.
Keywords: Adalimumab; Clinical pharmacology; Crohn’s disease; Peri-anal disorders.
©The Author(s) 2022. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: Sirmai L reports having received grant support from Abbvie and congress invitations from Roche and Sandoz and having received conference or consultancy fees from Gilead, MSD, Abbvie, Mayoly Spindler, Takeda, Ipsen, Allergan France and Ferring; Pelletier AL reports having received grant support from Abbvie and financial support from Ferring; Zallot C reports having received financial support from Takeda, Abbvie, Ferring, Janssen and Pfizer; Bouguen G reports having received lecture fees from Abbvie, Ferring, MSD, Takeda and Pfizer and consultant fees from Takeda, Janssen, Mylan and Abbvie; Bouchard D reports having received speaking fees from Abbvie, MSD and Janssen, consultancy fees from Takeda, and congress invitations from Abbvie, Pfizer and Takeda; Gault N, Roland Nicaise P, Peyneau M, Sironneau S, Bittencourt M, Petitcollin A, Fernandez P all declare they have no conflicts of interest; Roblin X reports having received financial support from Abbvie, Amgen, Pfizer, Takeda, Janssen, MSD and Theradiag; Siproudhis L reports having received conference or consultancy fees from Gilead, MSD, Abbvie, Mayoly Spindler, Takeda, Ipsen, Allergan France and Ferring; Abramowitz L reports having received grant support from Abbvie and financial support from Takeda.
Figures
Similar articles
-
Effectiveness of adalimumab in perianal fistulas in crohn's disease patients naive to anti-TNF therapy.J Clin Gastroenterol. 2015 Jan;49(1):34-40. doi: 10.1097/MCG.0000000000000169. J Clin Gastroenterol. 2015. PMID: 25485513
-
Higher anti-TNF serum levels are associated with perianal fistula closure in Crohn's disease patients.Scand J Gastroenterol. 2019 Apr;54(4):453-458. doi: 10.1080/00365521.2019.1600014. Epub 2019 Apr 28. Scand J Gastroenterol. 2019. PMID: 31032686
-
Higher anti-tumor necrosis factor levels are associated with perianal fistula healing and fistula closure in Crohn's disease.Eur J Gastroenterol Hepatol. 2020 Jan;32(1):32-37. doi: 10.1097/MEG.0000000000001561. Eur J Gastroenterol Hepatol. 2020. PMID: 31567638
-
Strategies to Distinguish Perianal Fistulas Related to Crohn's Disease From Cryptoglandular Disease: Systematic Review With Meta-Analysis.Inflamm Bowel Dis. 2022 Sep 1;28(9):1363-1374. doi: 10.1093/ibd/izab286. Inflamm Bowel Dis. 2022. PMID: 34792583
-
Optimized timing of using infliximab in perianal fistulizing Crohn's disease.World J Gastroenterol. 2020 Apr 14;26(14):1554-1563. doi: 10.3748/wjg.v26.i14.1554. World J Gastroenterol. 2020. PMID: 32327905 Free PMC article. Review.
Cited by
-
Enterocutaneous Fistula in a Patient with Crohn's Disease After Internalization of a Foreign Body into the Gastrointestinal Tract.J Clin Med. 2025 Mar 28;14(7):2327. doi: 10.3390/jcm14072327. J Clin Med. 2025. PMID: 40217777 Free PMC article.
-
Perianal disease in inflammatory bowel disease: Broadening treatment and surveillance strategies for anal cancer.World J Gastroenterol. 2024 Jul 28;30(28):3373-3385. doi: 10.3748/wjg.v30.i28.3373. World J Gastroenterol. 2024. PMID: 39091713 Free PMC article. Review.
-
Treatment With Adalimumab 80 mg Every Other Week in Inflammatory Bowel Disease: Results of Treatment Intensification in Clinical Practice.Crohns Colitis 360. 2023 Jan 10;5(1):otac051. doi: 10.1093/crocol/otac051. eCollection 2023 Jan. Crohns Colitis 360. 2023. PMID: 36785555 Free PMC article.
-
Current and emerging therapeutic strategies for perianal fistula in Crohn's disease patients.Adv Pharmacol. 2024;101:159-182. doi: 10.1016/bs.apha.2024.10.013. Epub 2024 Oct 24. Adv Pharmacol. 2024. PMID: 39521599 Review.
References
-
- Schwartz DA, Loftus EV Jr, Tremaine WJ, Panaccione R, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of fistulizing Crohn's disease in Olmsted County, Minnesota. Gastroenterology. 2002;122:875–880. - PubMed
-
- Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, Podolsky DK, Sands BE, Braakman T, DeWoody KL, Schaible TF, van Deventer SJ. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398–1405. - PubMed
-
- Lichtiger S, Binion DG, Wolf DC, Present DH, Bensimon AG, Wu E, Yu AP, Cardoso AT, Chao J, Mulani PM, Lomax KG, Kent JD. The CHOICE trial: adalimumab demonstrates safety, fistula healing, improved quality of life and increased work productivity in patients with Crohn's disease who failed prior infliximab therapy. Aliment Pharmacol Ther. 2010;32:1228–1239. - PubMed
-
- Bouchard D, Abramowitz L, Bouguen G, Brochard C, Dabadie A, de Parades V, Eléouet-Kaplan M, Fathallah N, Faucheron JL, Maggiori L, Panis Y, Pigot F, Rouméguère P, Sénéjoux A, Siproudhis L, Staumont G, Suduca JM, Vinson-Bonnet B, Zeitoun JD. Anoperineal lesions in Crohn's disease: French recommendations for clinical practice. Tech Coloproctol. 2017;21:683–691. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials