Formation of organoid-like structures in the decellularized rat testis
- PMID: 35317108
- PMCID: PMC8917852
- DOI: 10.22038/IJBMS.2021.58294.12948
Formation of organoid-like structures in the decellularized rat testis
Abstract
Objectives: In testis, the extracellular matrix (ECM) in addition to the supportive role for cells in the seminiferous epithelium, is also essential for the accurate functioning of these cells. Thus, using a decellularized testicular ECM (DTECM), as a scaffold for three-dimensional (3D) culture of testicular cells can mimic native ECM for studying in vitro spermatogenesis.
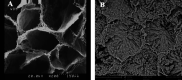
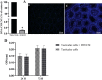
Materials and methods: The rat testis was decellularized via perfusion of 0.5% sodium dodecyl sulfate (SDS) for 48 hr, followed by 1% Triton X-100 for 6 hr, and then 1% DNase I for 1 hr. The efficiency of decellularization was evaluated by histology, immunohistochemistry (IHC), scanning electron microscopy (SEM), and MTT test. The prepared scaffolds were recellularized with testicular cells and cultured and assessed with hematoxylin-eosin (H&E) staining after two weeks.
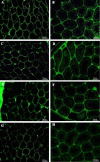
Results: Based on the H&E image, no trace of cell components could be observed in DTECM. IHC images demonstrated collagen types I and IV, laminin, and fibronectin were preserved. Masson's trichrome and alcian blue staining revealed that collagen and glycosaminoglycans (GAGs) were retained, and the SEM image indicated that 3D testicular architecture remained after the decellularization process. Based on the results of the MTT test, DTECM was cytocompatible, and H&E images represented that DTECM supports testicular cell arrangements in seminiferous tubule-like structures (STLSs) and organoid-like structures (OLSs).
Conclusion: The results showed that the applied protocol successfully decellularized the testis tissue of the rat. Therefore, these scaffolds may provide an appropriate vehicle for in vitro reconstruction of the seminiferous tubule.
Keywords: Decellularization; Extracellular matrix; Organoid; Seminiferous tubule; Testis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
The Decellularized Calf Testis: Introducing Suitable Scaffolds for Spermatogenesis Studies.Int J Fertil Steril. 2023 Nov 7;18(1):32-39. doi: 10.22074/ijfs.2023.1989173.1433. Int J Fertil Steril. 2023. PMID: 38041457 Free PMC article.
-
Three-Dimensional Culture of Mouse Spermatogonial Stem Cells Using A Decellularised Testicular Scaffold.Cell J. 2020 Jan;21(4):410-418. doi: 10.22074/cellj.2020.6304. Epub 2019 Jul 29. Cell J. 2020. PMID: 31376322 Free PMC article.
-
Derivation and characterization of a cytocompatible scaffold from human testis.Hum Reprod. 2015 Feb;30(2):256-67. doi: 10.1093/humrep/deu330. Epub 2014 Dec 11. Hum Reprod. 2015. PMID: 25505010
-
Characterization, recellularization, and transplantation of rat decellularized testis scaffold with bone marrow-derived mesenchymal stem cells.Stem Cell Res Ther. 2018 Nov 21;9(1):324. doi: 10.1186/s13287-018-1062-3. Stem Cell Res Ther. 2018. PMID: 30463594 Free PMC article.
-
Differentiation and proliferation of spermatogonial stem cells using a three-dimensional decellularized testicular scaffold: a new method to study the testicular microenvironment in vitro.Int Urol Nephrol. 2021 Aug;53(8):1543-1550. doi: 10.1007/s11255-021-02877-9. Epub 2021 May 11. Int Urol Nephrol. 2021. PMID: 33974223
Cited by
-
Engineering a 3D platform for testis bioengineering: generation and proteomic profiling of decellularized fish testicular scaffolds.Front Bioeng Biotechnol. 2025 Jul 25;13:1631542. doi: 10.3389/fbioe.2025.1631542. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40787197 Free PMC article.
-
Generation and Characterization of Bovine Testicular Organoids Derived from Primary Somatic Cell Populations.Animals (Basel). 2022 Sep 3;12(17):2283. doi: 10.3390/ani12172283. Animals (Basel). 2022. PMID: 36078004 Free PMC article.
-
Transcriptome Studies Reveal the N6-Methyladenosine Differences in Testis of Yaks at Juvenile and Sexual Maturity Stages.Animals (Basel). 2023 Sep 5;13(18):2815. doi: 10.3390/ani13182815. Animals (Basel). 2023. PMID: 37760215 Free PMC article.
-
The Decellularized Calf Testis: Introducing Suitable Scaffolds for Spermatogenesis Studies.Int J Fertil Steril. 2023 Nov 7;18(1):32-39. doi: 10.22074/ijfs.2023.1989173.1433. Int J Fertil Steril. 2023. PMID: 38041457 Free PMC article.
-
Biomaterials for Testicular Bioengineering: How far have we come and where do we have to go?Front Endocrinol (Lausanne). 2023 Mar 16;14:1085872. doi: 10.3389/fendo.2023.1085872. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37008920 Free PMC article. Review.
References
-
- Gulkesen K, Erdogru T, Sargin CF, Karpuzoglu G. Expression of extracellular matrix proteins and vimentin in testes of azoospermic man: an immunohistochemical and morphometric study. Asian J Androl. 2002;4:55–60. - PubMed
-
- Oğuzkurt P, Kayaselçuk F, Tuncer İ, Alkan M, Hiçsönmez A. Evaluation of extracellular matrix protein composition in sacs associated with undescended testis, hydrocele, inguinal hernia, and peritoneum. Urology. 2007;70:346–350. - PubMed
LinkOut - more resources
Full Text Sources
