Zerumbone mediates apoptosis and induces secretion of proinflammatory cytokines in breast carcinoma cell culture
- PMID: 35317109
- PMCID: PMC8917841
- DOI: 10.22038/IJBMS.2021.58573.13012
Zerumbone mediates apoptosis and induces secretion of proinflammatory cytokines in breast carcinoma cell culture
Abstract
Objectives: To investigate the potential anti-breast cancer activity of zerumbone in regulating apoptotic mediators and cytokines in comparison with paclitaxel (positive control).
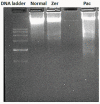
Materials and methods: In this study, assays such as viability, apoptosis, reactive oxygen species, cell cycle, DNA fragmentation, and cytokines were carried out on MCF-7 cells after treatment with zerumbone and paclitaxel.
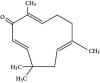
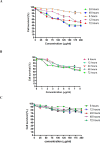
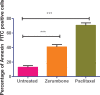
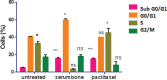
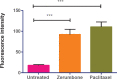
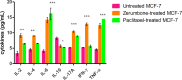
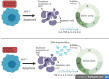
Results: The results showed that zerumbone demonstrated a higher (18-fold) IC50 value (126.7 µg/ml) than paclitaxel (7.29 µg/ml) in order to suppress proliferation and induce cell death of MCF-7. The cell cycle arrest at the G0/G1 phase and excessive intracellular ROS production during the in vitro zerumbone treatment indicated occurrence of apoptotic cell death although nuclear DNA fragmentation was not observed. The flow cytometer analysis of treated cells revealed secretion of proinflammatory cytokines suggesting the potential immunomodulatory activity of zerumbone.
Conclusion: Although, zerumbone exhibited a higher IC50 value compared with paclitaxel yet its anticancer activity against MCF-7 cells is still parallel to paclitaxel hence zerumbone has the potential to be an antineoplastic agent in the treatment of breast cancer especially the luminal type A.
Keywords: Apoptosis; Breast; Cytokine; Natural; Zerumbone.
Conflict of interest statement
There are no potential conflicts of interest reported by any of the authors.
Figures
Similar articles
-
Cytotoxic Activity of Isoniazid Derivative in Human Breast Cancer Cells.In Vivo. 2021 Sep-Oct;35(5):2675-2685. doi: 10.21873/invivo.12551. In Vivo. 2021. PMID: 34410956 Free PMC article.
-
Zerumbone-induced reactive oxygen species-mediated oxidative stress re-sensitizes breast cancer cells to paclitaxel.Biotechnol Appl Biochem. 2023 Feb;70(1):28-37. doi: 10.1002/bab.2326. Epub 2022 Mar 25. Biotechnol Appl Biochem. 2023. PMID: 35240000
-
Promotion of p53 expression and reactive oxidative stress production is involved in zerumbone-induced cisplatin sensitization of non-small cell lung cancer cells.Biochimie. 2014 Dec;107 Pt B:257-62. doi: 10.1016/j.biochi.2014.09.001. Epub 2014 Sep 16. Biochimie. 2014. PMID: 25220870
-
Zerumbone induces mitochondria-mediated apoptosis via increased calcium, generation of reactive oxygen species and upregulation of soluble histone H2AX in K562 chronic myelogenous leukemia cells.Tumour Biol. 2015 Nov;36(11):8479-89. doi: 10.1007/s13277-015-3583-z. Epub 2015 May 31. Tumour Biol. 2015. PMID: 26026585
-
Evaluation of the cytotoxicity, cell-cycle arrest, and apoptotic induction by Euphorbia hirta in MCF-7 breast cancer cells.Pharm Biol. 2016 Jul;54(7):1223-36. doi: 10.3109/13880209.2015.1064451. Epub 2015 Jul 8. Pharm Biol. 2016. PMID: 26154521
Cited by
-
Functional analysis of the effects of propofol on tamoxifen‑resistant breast cancer cells: Insights into transcriptional regulation.Oncol Lett. 2025 Feb 21;29(4):194. doi: 10.3892/ol.2025.14940. eCollection 2025 Apr. Oncol Lett. 2025. PMID: 40041408 Free PMC article.
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;0:caac. - PubMed
LinkOut - more resources
Full Text Sources
