Effect of Bifidobacterium longum 35624 on disease severity and quality of life in patients with irritable bowel syndrome
- PMID: 35317278
- PMCID: PMC8891724
- DOI: 10.3748/wjg.v28.i7.732
Effect of Bifidobacterium longum 35624 on disease severity and quality of life in patients with irritable bowel syndrome
Abstract
Background: Bifidobacterium longum 35624 has shown efficacy in improving irritable bowel syndrome (IBS) symptoms compared with placebo in double-blind randomized studies. However, few data are available from real-life clinical practice or from studies that used Rome IV criteria to diagnose IBS.
Aim: To assess the effect of B. longum 35624 on IBS severity and quality of life in a real-life setting.
Methods: From November 2018 to January 2020, 278 patients with IBS (according to Rome IV criteria) were enrolled in a prospective, open-label, multicenter observational study by private practice gastroenterologists to received one capsule of B. longum 35624 (109 colony-forming units) per day for 30 d. Participation in the study was independently proposed to patients during spontaneous consultations. Disease severity (assessed by the IBS severity scoring system) and patient quality of life (assessed by the IBS quality of life questionnaire) were compared between the inclusion visit (baseline) and the visit at the end of 30 d of treatment. The characteristics of patients were described at baseline. Continuous variables comparisons between inclusion and end-of-treatment visits were performed using the t-test and Kruskal-Wallis test. Categorical variables comparisons were performed using the χ2 test.
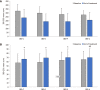
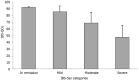
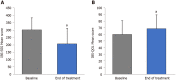
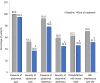
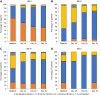
Results: A total of 233 patients, with a mean age of 51.4 years and composed of 71.2% women, were included in the study. Of these patients, 48.1% had moderate IBS and 46.4% had severe IBS. After a 30-d treatment period with one B. longum 35624 capsule per day, a significant decrease in IBS severity was observed compared to baseline (mean ± SD, IBS severity scoring system scores: 208 ± 104 vs 303 ± 81, P < 0.001) and 57% of patients moved to lower severity categories or achieved remission. The quality of life of patients was also improved by the treatment (IBS Quality of Life questionnaire score: 68.8 ± 20.9 vs 60.2 ± 20.5; P < 0.001) and 63.8% of patients were satisfied with the treatment.
Conclusion: Thirty days of treatment with B. longum 35624 reduces disease severity and improves the quality of life of patients with IBS, particularly those with the most severe forms of IBS.
Keywords: Abdominal pain; Bifidobacterium longum; Irritable bowel syndrome; Probiotics; Quality of life; Severity of illness index.
©The Author(s) 2022. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: Sabaté JM and Iglicki F report personal fees from Biocodex during the conduct of the study. Sabaté JM also reports personal fees from Biocodex, Kyowa Kirin, Norgine, Mayoly Spindler, Arko Pharma, and Tillots Pharma outside the submitted work.
Figures

Similar articles
-
Efficacy of Lactobacillus paracasei HA-196 and Bifidobacterium longum R0175 in Alleviating Symptoms of Irritable Bowel Syndrome (IBS): A Randomized, Placebo-Controlled Study.Nutrients. 2020 Apr 21;12(4):1159. doi: 10.3390/nu12041159. Nutrients. 2020. PMID: 32326347 Free PMC article. Clinical Trial.
-
A randomized double-blind, placebo-controlled trial to evaluate the safety and efficacy of live Bifidobacterium longum CECT 7347 (ES1) and heat-treated Bifidobacterium longum CECT 7347 (HT-ES1) in participants with diarrhea-predominant irritable bowel syndrome.Gut Microbes. 2024 Jan-Dec;16(1):2338322. doi: 10.1080/19490976.2024.2338322. Epub 2024 Apr 17. Gut Microbes. 2024. PMID: 38630015 Free PMC article. Clinical Trial.
-
[Long-term probiotic administration for irritable bowel syndrome: a legal need].Ter Arkh. 2023 Oct 11;95(8):679-685. doi: 10.26442/00403660.2023.08.202378. Ter Arkh. 2023. PMID: 38158905 Russian.
-
Bifidobacterium longum W11: Uniqueness and individual or combined clinical use in association with rifaximin.Clin Nutr ESPEN. 2021 Apr;42:15-21. doi: 10.1016/j.clnesp.2020.12.025. Epub 2021 Feb 17. Clin Nutr ESPEN. 2021. PMID: 33745570 Review.
-
The Effect of Bifidobacterium on Reducing Symptomatic Abdominal Pain in Patients with Irritable Bowel Syndrome: A Systematic Review.Probiotics Antimicrob Proteins. 2020 Sep;12(3):834-839. doi: 10.1007/s12602-019-09609-7. Probiotics Antimicrob Proteins. 2020. PMID: 31741311 Free PMC article.
Cited by
-
Lactobacillus gasseri LA806 Supplementation in Patients with Irritable Bowel Syndrome: A Multicenter Study.J Clin Med. 2022 Dec 15;11(24):7446. doi: 10.3390/jcm11247446. J Clin Med. 2022. PMID: 36556059 Free PMC article.
-
Altered gut microbiota in the early stage of acute pancreatitis were related to the occurrence of acute respiratory distress syndrome.Front Cell Infect Microbiol. 2023 Mar 6;13:1127369. doi: 10.3389/fcimb.2023.1127369. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36949815 Free PMC article.
-
Compendium of Bifidobacterium-based probiotics: characteristics and therapeutic impact on human diseases.Microbiome Res Rep. 2024 Oct 8;4(1):2. doi: 10.20517/mrr.2024.52. eCollection 2025. Microbiome Res Rep. 2024. PMID: 40207278 Free PMC article. Review.
-
Probiotic Potential in Irritable Bowel Syndrome and Inflammatory Bowel Disease: A Comprehensive Systematic Review.Cureus. 2024 Oct 22;16(10):e72089. doi: 10.7759/cureus.72089. eCollection 2024 Oct. Cureus. 2024. PMID: 39575029 Free PMC article. Review.
-
Management of irritable bowel syndrome: a narrative review.Transl Gastroenterol Hepatol. 2024 Mar 21;9:26. doi: 10.21037/tgh-23-96. eCollection 2024. Transl Gastroenterol Hepatol. 2024. PMID: 38716216 Free PMC article. Review.
References
-
- Sperber AD, Bangdiwala SI, Drossman DA, Ghoshal UC, Simren M, Tack J, Whitehead WE, Dumitrascu DL, Fang X, Fukudo S, Kellow J, Okeke E, Quigley EMM, Schmulson M, Whorwell P, Archampong T, Adibi P, Andresen V, Benninga MA, Bonaz B, Bor S, Fernandez LB, Choi SC, Corazziari ES, Francisconi C, Hani A, Lazebnik L, Lee YY, Mulak A, Rahman MM, Santos J, Setshedi M, Syam AF, Vanner S, Wong RK, Lopez-Colombo A, Costa V, Dickman R, Kanazawa M, Keshteli AH, Khatun R, Maleki I, Poitras P, Pratap N, Stefanyuk O, Thomson S, Zeevenhooven J, Palsson OS. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology. 2021;160:99–114.e3. - PubMed
-
- Drossman DA, Chang L, Bellamy N, Gallo-Torres HE, Lembo A, Mearin F, Norton NJ, Whorwell P. Severity in irritable bowel syndrome: a Rome Foundation Working Team report. Am J Gastroenterol. 2011;106:1749–59; quiz 1760. - PubMed
-
- Ford AC, Lacy BE, Talley NJ. Irritable Bowel Syndrome. N Engl J Med. 2017;376:2566–2578. - PubMed
-
- Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48:1044–1060. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

