Humoral responses after second and third SARS-CoV-2 vaccination in patients with immune-mediated inflammatory disorders on immunosuppressants: a cohort study
- PMID: 35317410
- PMCID: PMC8930018
- DOI: 10.1016/S2665-9913(22)00034-0
Humoral responses after second and third SARS-CoV-2 vaccination in patients with immune-mediated inflammatory disorders on immunosuppressants: a cohort study
Abstract
Background: Disease-specific studies have reported impaired humoral responses after SARS-CoV-2 vaccination in patients with immune-mediated inflammatory disorders treated with specific immunosuppressants. Disease-overarching studies, and data on recall responses and third vaccinations are scarce. Our primary objective was to investigate the effects of immunosuppressive monotherapies on the humoral immune response after SARS-CoV-2 vaccination in patients with prevalent immune-mediated inflammatory disorders.
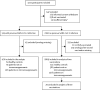
Methods: We did a cohort study in participants treated in outpatient clinics in seven university hospitals and one rheumatology treatment centre in the Netherlands as well as participants included in two national cohort studies on COVID-19-related disease severity. We included patients aged older than 18 years, diagnosed with any of the prespecified immune-mediated inflammatory disorders, who were able to understand and complete questionnaires in Dutch. Participants with immune-mediated inflammatory disorders who were not on systemic immunosuppressants and healthy participants were included as controls. Anti-receptor binding domain IgG responses and neutralisation capacity were monitored following standard vaccination regimens and a three-vaccination regimen in subgroups. Hybrid immune responses-ie, vaccination after previous SARS-CoV-2 infection-were studied as a proxy for recall responses.
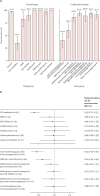
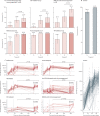
Findings: Between Feb 2 and Aug 1, 2021, we included 3222 participants in our cohort. Sera from 2339 participants, 1869 without and 470 participants with previous SARS-CoV-2 infection were analysed (mean age 49·9 years [SD 13·7]; 1470 [62·8%] females and 869 [37·2%] males). Humoral responses did not differ between disorders. Anti-CD20 therapy, sphingosine 1-phosphate receptor (S1P) modulators, and mycophenolate mofetil combined with corticosteroids were associated with lower relative risks for reaching seroconversion following standard vaccination (0·32 [95% CI 0·19-0·49] for anti-CD20 therapy, 0·35 [0·21-0·55] for S1P modulators, and 0·61 [0·40-0·90] for mycophenolate mofetil combined with corticosteroids). A third vaccination increased seroconversion for mycophenolate mofetil combination treatments (from 52·6% after the second vaccination to 89·5% after the third) but not significantly for anti-CD20 therapies (from 36·8% to 45·6%) and S1P modulators (from 35·5% to 48·4%). Most other immunosuppressant groups showed moderately reduced antibody titres after standard vaccination that did not increase after a third vaccination, although seroconversion rates and neutralisation capacity were unaffected. In participants with previous SARS-CoV-2 infection, SARS-CoV-2 antibodies were boosted after vaccination, regardless of immunosuppressive treatment.
Interpretation: Humoral responses following vaccination are impaired by specific immunosuppressants. After standard vaccination regimens, patients with immune-mediated inflammatory disorders taking most immunosuppressants show similar seroconversion to controls, although antibody titres might be moderately reduced. As neutralisation capacity and recall responses are also preserved in these patients, this is not likely to translate to loss of (short-term) protection. In patients on immunosuppressants showing poor humoral responses after standard vaccination regimens, a third vaccination resulted in additional seroconversion in patients taking mycophenolate mofetil combination treatments, whereas the effect of a third vaccination in patients on anti-CD20 therapy and S1P modulators was limited.
Funding: ZonMw (The Netherlands Organization for Health Research and Development).
© 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
FE and TWK report (governmental) grants from ZonMw to study immune response after SARS-Cov-2 vaccination in autoimmune diseases. FE also reports grants from Prinses Beatrix Spierfonds, CSL Behring, Kedrion, Terumo BCT, Grifols, Takeda Pharmaceutical Company, and GBS-CIDP Foundation; consulting fees from UCB Pharma and CSL Behring; and honoraria from Grifols. AJvdK reports grants from CSL Behring and participation on an advisory board for Argen-X. ML reports a grant from Galapagos not related to this study, and honoraria from Bristol Myers Squibb, Pfizer, Takeda, and Tillotts. PIS is involved in clinical trials with many pharmaceutical industries that manufacture drugs used for the treatment of, for example, psoriasis and atopic dermatitis, for which financial compensation is paid to the department or hospital, and is a chief investigator of the TREAT NL registry taskforce and SECURE-AD registry. MWB is a secretary for the Dutch Experimental Dermatology Board; head of the pigmentary disorders group within the Dutch Dermatology Board; and reports honoraria from Pfizer, Sanofi, Novartis, and Fondation René Touraine. JK has speaking relationships with Merck Serono, Biogen Idec, TEVA, Sanofi, Genzyme, Roche, and Novartis; received financial support to his institution for research activities from Merck Serono, Bayer Shcering Pharma, Biogen Idec, GlaxoSmithKline (GSK), Roche, Teva, Sanofi, Genzyme, and Novartis. BH reports unpaid positions as a medical adviser for several patient groups, a board position for ERN-SKIN, and associate editor for The British Journal of Dermatology; reports grants from AbbVie, Akari Therapeutics, Celgene, and Novartis; consulting fees from UCB Pharma, Novartis, and Janssen; and honoraria from AbbVie. JJGMV reports consulting fees from Argenx, Alexion, and NMD Pharma, and is a co-inventor on patent applications based on MuSK protein-related research. DJH reports grants from AbbVie, AstraZeneca, Janssen, LEO Pharma, and UCB; honoraria from AbbVie, Galderma, Janssen, Lilly, Pfizer, Sanofi, and UCB; and a paid position on an advisory board for BIOMAP IMI. PAvD participated on an advisory board for Octapharma. PvP reports grants from Alexion Pharma and GSK, and participation on advisory boards for GSK and Vifor Pharma. GRAMD'H reports consulting fees from AbbVie, Agomab, AstraZeneca, AM Pharma, AMT, Arena Pharmaceuticals, Bristol Myers Squibb, Boehringer Ingelheim, Celltrion, Eli Lilly, Exeliom Biosciences, Exo Biologics, Galapagos, Index Pharmaceuticals, Kaleido, Roche, Gilead, GSK, Gossamerbio, Pfizer, Immunic, Johnson and Johnson, Origo, Polpharma, Procise Diagnostics, Prometheus Laboratories, Prometheus Biosciences, Progenity, and Protagonist; honoraria from AbbVie, Arena, Galapagos, Gilead, Pfizer, Bristol Myers Squibb, and Takeda; and participation on advisory boards for AbbVie, Seres Health, Galapagos, and AstraZeneca. RBT reports honoraria from Sobi and Norgine, and participation on an advisory board for Norgine. HSG is a board member of the Dutch Society of Clinical Neurophysiology (unpaid), reports grants from Prinses Beatrix Spierfonds, and received speaker fees from Shire/Takeda. KAHZ reports paid data safety monitoring board positions for Torrent and Foresee. All other authors declare no competing interests.
Figures



References
-
- Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330–1338. - PubMed
-
- Braun-Moscovici Y, Kaplan M, Braun M, et al. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the Pfizer mRNA vaccine against SARS-CoV-2. Ann Rheum Dis. 2021;80:1317–1321. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

