Adaptive evolution of West Nile virus facilitated increased transmissibility and prevalence in New York State
- PMID: 35317702
- PMCID: PMC8982463
- DOI: 10.1080/22221751.2022.2056521
Adaptive evolution of West Nile virus facilitated increased transmissibility and prevalence in New York State
Abstract
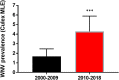
West Nile virus (WNV; Flavivirus, Flaviviridae) was introduced to New York State (NYS) in 1999 and rapidly expanded its range through the continental United States (US). Apart from the displacement of the introductory NY99 genotype with the WN02 genotype, there has been little evidence of adaptive evolution of WNV in the US. WNV NY10, characterized by shared amino acid substitutions R1331K and I2513M, emerged in 2010 coincident with increased WNV cases in humans and prevalence in mosquitoes. Previous studies demonstrated an increase in frequency of NY10 strains in NYS and evidence of positive selection. Here, we present updated surveillance and sequencing data for WNV in NYS and investigate if NY10 genotype strains are associated with phenotypic change consistent with an adaptive advantage. Results confirm a significant increase in prevalence in mosquitoes though 2018, and updated sequencing demonstrates a continued dominance of NY10. We evaluated NY10 strains in Culex pipiens mosquitoes to assess vector competence and found that the NY10 genotype is associated with both increased infectivity and transmissibility. Experimental infection of American robins (Turdus migratorius) was additionally completed to assess viremia kinetics of NY10 relative to WN02. Modelling the increased infectivity and transmissibility of the NY10 strains together with strain-specific viremia demonstrates a mechanistic basis for selection that has likely contributed to the increased prevalence of WNV in NYS.
Keywords: Flavivirus; West Nile virus; adaptive evolution; arbovirus; virus evolution.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




Similar articles
-
Rising temperatures contribute to West Nile virus diversification and increased transmission potential.Sci Rep. 2025 Jul 11;15(1):25016. doi: 10.1038/s41598-025-09284-5. Sci Rep. 2025. PMID: 40646045 Free PMC article.
-
Noncoding Subgenomic Flavivirus RNA Is Processed by the Mosquito RNA Interference Machinery and Determines West Nile Virus Transmission by Culex pipiens Mosquitoes.J Virol. 2016 Oct 28;90(22):10145-10159. doi: 10.1128/JVI.00930-16. Print 2016 Nov 15. J Virol. 2016. PMID: 27581979 Free PMC article.
-
Vector competence of Argentine mosquitoes (Diptera: Culicidae) for West Nile virus (Flaviviridae: Flavivirus).J Med Entomol. 2013 Jul;50(4):853-62. doi: 10.1603/me12226. J Med Entomol. 2013. PMID: 23926785 Free PMC article.
-
Molecular epidemiology and evolution of West Nile virus in North America.Int J Environ Res Public Health. 2013 Oct 16;10(10):5111-29. doi: 10.3390/ijerph10105111. Int J Environ Res Public Health. 2013. PMID: 24135819 Free PMC article. Review.
-
On the Fly: Interactions Between Birds, Mosquitoes, and Environment That Have Molded West Nile Virus Genomic Structure Over Two Decades.J Med Entomol. 2019 Oct 28;56(6):1467-1474. doi: 10.1093/jme/tjz112. J Med Entomol. 2019. PMID: 31549720 Free PMC article. Review.
Cited by
-
Patterns of West Nile Virus in the Northeastern United States Using Negative Binomial and Mechanistic Trait-Based Models.Geohealth. 2023 Apr 4;7(4):e2022GH000747. doi: 10.1029/2022GH000747. eCollection 2023 Apr. Geohealth. 2023. PMID: 37026081 Free PMC article.
-
Surveillance and Genetic Analysis of Jamestown Canyon Virus in New York State: 2001-2022.Am J Trop Med Hyg. 2023 Oct 9;109(6):1329-1332. doi: 10.4269/ajtmh.23-0392. Print 2023 Dec 6. Am J Trop Med Hyg. 2023. PMID: 37972332 Free PMC article.
-
The impact of climate change on transmission season length: West Nile virus as a case study.bioRxiv [Preprint]. 2025 Aug 2:2025.08.01.667982. doi: 10.1101/2025.08.01.667982. bioRxiv. 2025. PMID: 40766431 Free PMC article. Preprint.
-
The emergence of NY10: insights into the 2012 West Nile Virus outbreak in the United States.Virus Evol. 2025 May 14;11(1):veaf037. doi: 10.1093/ve/veaf037. eCollection 2025. Virus Evol. 2025. PMID: 40574749 Free PMC article.
-
An advanced sequence clustering and designation workflow reveals the enzootic maintenance of a dominant West Nile virus subclade in Germany.Virus Evol. 2023 Mar 17;9(1):vead013. doi: 10.1093/ve/vead013. eCollection 2023. Virus Evol. 2023. PMID: 37197362 Free PMC article.
References
-
- Brinton MA. The molecular biology of West Nile virus: a new invader of the Western hemisphere. Annu Rev Microbiol. 2002;56:371–402. - PubMed
-
- Hayes CG. West Nile virus: Uganda, 1937, to New York City, 1999. Ann N Y Acad Sci. 2001;951:25–37. - PubMed
-
- Murgue B, Zeller H, Deubel V.. The ecology and epidemiology of West Nile virus in Africa, Europe and Asia. Curr Top Microbiol Immunol. 2002;267:195–221. - PubMed
-
- Lanciotti RS, Ebel GD, Deubel V, et al. . Complete genome sequences and phylogenetic analysis of West Nile virus strains isolated from the United States, Europe, and the Middle East. Virology. 2002;298(1):96–105. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
