Wound-induced signals regulate root organogenesis in Arabidopsis explants
- PMID: 35317749
- PMCID: PMC8939181
- DOI: 10.1186/s12870-022-03524-w
Wound-induced signals regulate root organogenesis in Arabidopsis explants
Erratum in
-
Correction: Wound-induced signals regulate root organogenesis in Arabidopsis explants.BMC Plant Biol. 2023 May 22;23(1):271. doi: 10.1186/s12870-023-04291-y. BMC Plant Biol. 2023. PMID: 37211591 Free PMC article. No abstract available.
Abstract
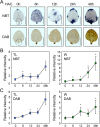
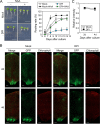
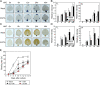
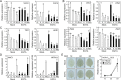
Background: Reactive oxygen species (ROS) and calcium ions (Ca2+) are representative signals of plant wound responses. Wounding triggers cell fate transition in detached plant tissues and induces de novo root organogenesis. While the hormonal regulation of root organogenesis has been widely studied, the role of early wound signals including ROS and Ca2+ remains largely unknown.
Results: We identified that ROS and Ca2+ are required for de novo root organogenesis, but have different functions in Arabidopsis explants. The inhibition of the ROS and Ca2+ signals delayed root development in detached leaves. Examination of the auxin signaling pathways indicated that ROS and Ca2+ did not affect auxin biosynthesis and transport in explants. Additionally, the expression of key genes related to auxin signals during root organogenesis was not significantly affected by the inhibition of ROS and Ca2+ signals. The addition of auxin partially restored the suppression of root development by the ROS inhibitor; however, auxin supplementation did not affect root organogenesis in Ca2+-depleted explants.
Conclusions: Our results indicate that, while both ROS and Ca2+ are key molecules, at least in part of the auxin signals acts downstream of ROS signaling, and Ca2+ acts downstream of auxin during de novo root organogenesis in leaf explants.
Keywords: Auxin; Calcium ion; Explants; ROS; Root organogenesis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
ENHANCER OF SHOOT REGENERATION1 promotes de novo root organogenesis after wounding in Arabidopsis leaf explants.Plant Cell. 2024 May 29;36(6):2359-2374. doi: 10.1093/plcell/koae074. Plant Cell. 2024. PMID: 38445764 Free PMC article.
-
YUCCA-mediated auxin biogenesis is required for cell fate transition occurring during de novo root organogenesis in Arabidopsis.J Exp Bot. 2016 Jul;67(14):4273-84. doi: 10.1093/jxb/erw213. Epub 2016 Jun 2. J Exp Bot. 2016. PMID: 27255928 Free PMC article.
-
Auxin-Independent NAC Pathway Acts in Response to Explant-Specific Wounding and Promotes Root Tip Emergence during de Novo Root Organogenesis in Arabidopsis.Plant Physiol. 2016 Apr;170(4):2136-45. doi: 10.1104/pp.15.01733. Epub 2016 Feb 5. Plant Physiol. 2016. PMID: 26850273 Free PMC article.
-
De novo root regeneration from leaf explants: wounding, auxin, and cell fate transition.Curr Opin Plant Biol. 2018 Feb;41:39-45. doi: 10.1016/j.pbi.2017.08.004. Epub 2017 Dec 11. Curr Opin Plant Biol. 2018. PMID: 28865805 Review.
-
Multiple layers of regulators emerge in the network controlling lateral root organogenesis.Trends Plant Sci. 2025 May;30(5):499-514. doi: 10.1016/j.tplants.2024.09.018. Epub 2024 Oct 24. Trends Plant Sci. 2025. PMID: 39455398 Review.
Cited by
-
ROS-mediated plasmodesmal regulation requires a network of an Arabidopsis receptor-like kinase, calmodulin-like proteins, and callose synthases.Front Plant Sci. 2023 Jan 19;13:1107224. doi: 10.3389/fpls.2022.1107224. eCollection 2022. Front Plant Sci. 2023. PMID: 36743578 Free PMC article.
-
ENHANCER OF SHOOT REGENERATION1 promotes de novo root organogenesis after wounding in Arabidopsis leaf explants.Plant Cell. 2024 May 29;36(6):2359-2374. doi: 10.1093/plcell/koae074. Plant Cell. 2024. PMID: 38445764 Free PMC article.
-
ESR2-HDA6 complex negatively regulates auxin biosynthesis to delay callus initiation in Arabidopsis leaf explants during tissue culture.Plant Commun. 2024 Jul 8;5(7):100892. doi: 10.1016/j.xplc.2024.100892. Epub 2024 Apr 2. Plant Commun. 2024. PMID: 38566417 Free PMC article.
-
Correction: Wound-induced signals regulate root organogenesis in Arabidopsis explants.BMC Plant Biol. 2023 May 22;23(1):271. doi: 10.1186/s12870-023-04291-y. BMC Plant Biol. 2023. PMID: 37211591 Free PMC article. No abstract available.
-
Free Radicals Mediated Redox Signaling in Plant Stress Tolerance.Life (Basel). 2023 Jan 10;13(1):204. doi: 10.3390/life13010204. Life (Basel). 2023. PMID: 36676153 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

