Synthesis, crystal structure, Hirshfeld surface investigation and comparative DFT studies of ethyl 2-[2-(2-nitrobenzylidene)hydrazinyl]thiazole-4-carboxylate
- PMID: 35317817
- PMCID: PMC8941777
- DOI: 10.1186/s13065-022-00805-1
Synthesis, crystal structure, Hirshfeld surface investigation and comparative DFT studies of ethyl 2-[2-(2-nitrobenzylidene)hydrazinyl]thiazole-4-carboxylate
Abstract
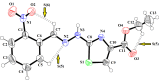
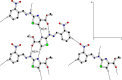
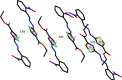
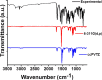
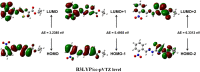
The ethyl 2-[2-(2-nitrobenzylidene)hydrazinyl]thiazole-4-carboxylate (1), a thiazole ester, was synthesized by refluxing 1-(2-nitrobenzylidene)thiosemicarbazide and ethyl bromopyruvate. The compound is characterized by spectrometric, spectroscopic and single crystal (SC-XRD) techniques. Non-covalent interactions that are responsible for crystal packing are explored by Hirshfeld surface analysis. All theoretical calculations were performed by DFT quantum chemical methods using 6-311G(d,p) and cc-pVTZ basis sets and compared. Theoretical harmonic frequencies of ethyl 2-[2-(2-nitrobenzylidene)hydrazinyl]thiazole-4-carboxylate (1) were optimized. Confirmation of hydrogen bonding sites was analyzed by molecular electrostatic potential (MEP) and Mulliken population analysis. The vibrational frequencies of characteristic functional groups and chemical shifts were found in good agreement with experimental assignments. Frontier molecular orbital (FMO) revealed relatively small HOMO-LUMO (highest occupied molecular orbital-lowest unoccupied molecular orbital) gape, which speaks off the nearly planar geometry and extended conjugation, as compared to the substituents with no conjugation possible. It has also been observed that -NO2 substituent plays a vital role for this relatively small HOMO-LUMO gape and overall electronic properties when compared with similar thiazole carboxylates (2-6, Table 6). Ethyl 2-[2-(2-nitrobenzylidene)hydrazinyl]thiazole-4-carboxylate (1) was also evaluated for its anti-oxidant and anti-microbial activities.
Keywords: Anti-oxidant; DFT calculations; Spectroscopy; Thiazole; XRD.
© 2022. The Author(s).
Conflict of interest statement
No competing interests.
Figures
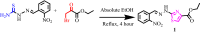









Similar articles
-
Synthesis, antioxidant, antimicrobial and antiviral docking studies of ethyl 2-(2-(arylidene)hydrazinyl)thiazole-4-carboxylates.Z Naturforsch C J Biosci. 2021 Apr 26;76(11-12):467-480. doi: 10.1515/znc-2021-0042. Print 2021 Nov 25. Z Naturforsch C J Biosci. 2021. PMID: 33901389
-
Experimental spectral characterization, Hirshfeld surface analysis, DFT/TD-DFT calculations and docking studies of (2Z,5Z)-5-(4-nitrobenzylidene)-3-N(2-methoxyphenyl)-2-N'(2-methoxyphenylimino) thiazolidin-4-one.Heliyon. 2020 Dec 22;6(12):e05754. doi: 10.1016/j.heliyon.2020.e05754. eCollection 2020 Dec. Heliyon. 2020. PMID: 33385082 Free PMC article.
-
Theoretical Investigation of para Amino-Dichloro Chalcone Isomers. Part II: A DFT Structure-Stability Study of the FMO and NLO Properties.ACS Omega. 2023 Jan 27;8(5):4937-4953. doi: 10.1021/acsomega.2c07148. eCollection 2023 Feb 7. ACS Omega. 2023. PMID: 36777615 Free PMC article.
-
Investigation of the molecular structure of 4-(3-methyl-3-phenylcyclobutyl)-2-[2-(3-methylbenzylidene)hydrazinyl]thiazole in the gas and solid phases.Acta Crystallogr C Struct Chem. 2018 Nov 1;74(Pt 11):1502-1508. doi: 10.1107/S2053229618013475. Epub 2018 Oct 23. Acta Crystallogr C Struct Chem. 2018. PMID: 30398207
-
Vibrational spectra (experimental and theoretical), molecular structure, natural bond orbital, HOMO-LUMO energy, Mulliken charge and thermodynamic analysis of N'-hydroxy-pyrimidine-2-carboximidamide by DFT approach.Spectrochim Acta A Mol Biomol Spectrosc. 2015 Jun 5;144:215-25. doi: 10.1016/j.saa.2015.02.100. Epub 2015 Feb 27. Spectrochim Acta A Mol Biomol Spectrosc. 2015. PMID: 25756689
Cited by
-
Synthesis, Characterization and DFT Study of a New Family of High-Energy Compounds Based on s-Triazine, Carborane and Tetrazoles.Molecules. 2022 Nov 2;27(21):7484. doi: 10.3390/molecules27217484. Molecules. 2022. PMID: 36364313 Free PMC article.
-
Exploring Highly Functionalized Tetrahydropyridine as a Dual Inhibitor of Monoamine Oxidase A and B: Synthesis, Structural Analysis, Single Crystal XRD, Supramolecular Assembly Exploration by Hirshfeld Surface Analysis, and Computational Studies.ACS Omega. 2022 Aug 11;7(33):29452-29464. doi: 10.1021/acsomega.2c03909. eCollection 2022 Aug 23. ACS Omega. 2022. PMID: 36033707 Free PMC article.
-
Design, and synthesis of selectively anticancer 4-cyanophenyl substituted thiazol-2-ylhydrazones.RSC Adv. 2022 Nov 28;12(52):34126-34141. doi: 10.1039/d2ra03226k. eCollection 2022 Nov 22. RSC Adv. 2022. PMID: 36540407 Free PMC article.
-
Synthesis, X-ray diffraction analysis, quantum chemical studies and α-amylase inhibition of probenecid derived S-alkylphthalimide-oxadiazole-benzenesulfonamide hybrids.J Enzyme Inhib Med Chem. 2022 Dec;37(1):1464-1478. doi: 10.1080/14756366.2022.2078969. J Enzyme Inhib Med Chem. 2022. PMID: 35616297 Free PMC article.
References
-
- Siddiqui N, Arshad MF, Ahsan W, Alam MS. Thiazoles: A valuable insight into the recent advances and biological activities. Int J Pharm Sci Drug Res. 2009;1(3):136–143.
-
- Hudson BM, Nguyen E, Tantillo DJ. The influence of intramolecular sulfur–lone pair interactions on small-molecule drug design and receptor binding. Org Biomol Chem. 2016;14:3975–3980. - PubMed
-
- Cunico W, Gomes CRB, Vellasco WTJ. Chemistry and Biological Activities of 1,3-Thiazolidin-4-ones. Mini Rev Org Chem. 2008;5:336–344.
-
- Verma A, Saraf SK. 4-thiazolidinone–a biologically active scaffold. Eur J Med Chem. 2008;43:897–905. - PubMed
-
- Hamama WS, Ismail MA, Shaaban S, Zoorob HHJ. Progress in the chemistry of 4- thiazolidinones. Heterocycl Chem. 2008;45(4):939–956.
LinkOut - more resources
Full Text Sources
