Synthesis, crystal structure, Hirshfeld surface investigation and comparative DFT studies of ethyl 2-[2-(2-nitrobenzylidene)hydrazinyl]thiazole-4-carboxylate
- PMID: 35317817
- PMCID: PMC8941777
- DOI: 10.1186/s13065-022-00805-1
Synthesis, crystal structure, Hirshfeld surface investigation and comparative DFT studies of ethyl 2-[2-(2-nitrobenzylidene)hydrazinyl]thiazole-4-carboxylate
Abstract
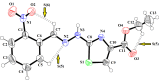
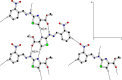
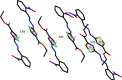
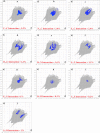
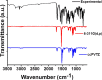
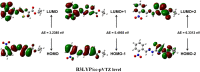
The ethyl 2-[2-(2-nitrobenzylidene)hydrazinyl]thiazole-4-carboxylate (1), a thiazole ester, was synthesized by refluxing 1-(2-nitrobenzylidene)thiosemicarbazide and ethyl bromopyruvate. The compound is characterized by spectrometric, spectroscopic and single crystal (SC-XRD) techniques. Non-covalent interactions that are responsible for crystal packing are explored by Hirshfeld surface analysis. All theoretical calculations were performed by DFT quantum chemical methods using 6-311G(d,p) and cc-pVTZ basis sets and compared. Theoretical harmonic frequencies of ethyl 2-[2-(2-nitrobenzylidene)hydrazinyl]thiazole-4-carboxylate (1) were optimized. Confirmation of hydrogen bonding sites was analyzed by molecular electrostatic potential (MEP) and Mulliken population analysis. The vibrational frequencies of characteristic functional groups and chemical shifts were found in good agreement with experimental assignments. Frontier molecular orbital (FMO) revealed relatively small HOMO-LUMO (highest occupied molecular orbital-lowest unoccupied molecular orbital) gape, which speaks off the nearly planar geometry and extended conjugation, as compared to the substituents with no conjugation possible. It has also been observed that -NO2 substituent plays a vital role for this relatively small HOMO-LUMO gape and overall electronic properties when compared with similar thiazole carboxylates (2-6, Table 6). Ethyl 2-[2-(2-nitrobenzylidene)hydrazinyl]thiazole-4-carboxylate (1) was also evaluated for its anti-oxidant and anti-microbial activities.
Keywords: Anti-oxidant; DFT calculations; Spectroscopy; Thiazole; XRD.
© 2022. The Author(s).
Conflict of interest statement
No competing interests.
Figures
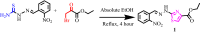









References
-
- Siddiqui N, Arshad MF, Ahsan W, Alam MS. Thiazoles: A valuable insight into the recent advances and biological activities. Int J Pharm Sci Drug Res. 2009;1(3):136–143.
-
- Hudson BM, Nguyen E, Tantillo DJ. The influence of intramolecular sulfur–lone pair interactions on small-molecule drug design and receptor binding. Org Biomol Chem. 2016;14:3975–3980. - PubMed
-
- Cunico W, Gomes CRB, Vellasco WTJ. Chemistry and Biological Activities of 1,3-Thiazolidin-4-ones. Mini Rev Org Chem. 2008;5:336–344.
-
- Verma A, Saraf SK. 4-thiazolidinone–a biologically active scaffold. Eur J Med Chem. 2008;43:897–905. - PubMed
-
- Hamama WS, Ismail MA, Shaaban S, Zoorob HHJ. Progress in the chemistry of 4- thiazolidinones. Heterocycl Chem. 2008;45(4):939–956.
