The effect of flexible low-dose GnRH antagonist on pregnancy outcome in the fresh embryo transfer cycle of IVF-ET: a randomized controlled trial
- PMID: 35317821
- PMCID: PMC8939190
- DOI: 10.1186/s12958-022-00927-0
The effect of flexible low-dose GnRH antagonist on pregnancy outcome in the fresh embryo transfer cycle of IVF-ET: a randomized controlled trial
Abstract
Objective: To explore the practicality and effectiveness of a flexible low-dose protocol in the fresh embryo transfer cycle: reducing the total amount of antagonist by increasing the interval between administrations of Cetrotide.
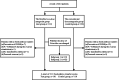
Methods: A total of 211 patients with normal ovarian reserve who accepted GnRH-ant protocol for IVF-ET were selected, and they were randomized to the flexible low-dose antagonist group (test group, n = 101) or the conventional dose antagonist group (control group, n = 110). The initial dose of Cetrotide in the test group was 0.25 mg every other day, and then the dose was adjusted to 0.25 mg every day based on the subsequent luteinizing hormone (LH) levels. The dosage of Cetrotide in the control group was 0.25 mg per day. The primary outcome was the clinical pregnancy rate. Secondary outcomes included the incidence of premature LH rise, total dosage of Cetrotide, number of oocytes retrieved, number of fertilized oocytes, number of high-quality embryos, biochemical pregnancy rate and ongoing pregnancy rate.
Results: There was no significant difference in the general condition of the two groups. There was no significant difference in the clinical pregnancy rate (51.49% vs. 48.18%, p = 0.632) or the incidence of premature LH rise (18.81% vs. 15.45%, p = 0.584) between the two groups. However, the amount of Cetrotide used in the test group was significantly lower than that in the conventional dose antagonist group (1.13 ± 0.41 vs. 1.61 ± 0.59 mg, p < 0.001).
Conclusion: The flexible low-dose antagonist protocol and the conventional dose antagonist protocol were equally effective in people with a normal ovarian reserve in the fresh embryo transfer cycle of IVF-ET.
Keywords: Cetrotide; Clinical pregnancy rate; Flexible low-dose GnRH antagonist; IVF-ET; Premature LH rise.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest. No potential conflicts of interest were disclosed.
Similar articles
-
Recombinant follicle-stimulating hormone and recombinant luteinizing hormone versus recombinant follicle-stimulating hormone alone during GnRH antagonist ovarian stimulation in patients aged ≥35 years: a randomized controlled trial.Hum Reprod. 2015 May;30(5):1188-95. doi: 10.1093/humrep/dev038. Epub 2015 Mar 3. Hum Reprod. 2015. PMID: 25740882 Clinical Trial.
-
Effect of Flexible Half-Dose Gonadotropin-Releasing Hormone Antagonist Protocol on in vitro Fertilization Outcome in Predicted Normal Responder: A Study Protocol for a Multicentered, Randomized, Non-Inferiority, Parallel Controlled Trial.Drug Des Devel Ther. 2023 May 24;17:1557-1566. doi: 10.2147/DDDT.S409557. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 37249929 Free PMC article.
-
Low Serum LH Levels During Ovarian Stimulation With GnRH Antagonist Protocol Decrease the Live Birth Rate After Fresh Embryo Transfers but Have No Impact in Freeze-All Cycles.Front Endocrinol (Lausanne). 2021 Apr 23;12:640047. doi: 10.3389/fendo.2021.640047. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33967956 Free PMC article.
-
Higher probability of live-birth in high, but not normal, responders after first frozen-embryo transfer in a freeze-only cycle strategy compared to fresh-embryo transfer: a meta-analysis.Hum Reprod. 2019 Mar 1;34(3):491-505. doi: 10.1093/humrep/dey388. Hum Reprod. 2019. PMID: 30689865
-
GnRH antagonists in ovarian stimulation for IVF.Hum Reprod Update. 2006 Jul-Aug;12(4):333-40. doi: 10.1093/humupd/dml001. Epub 2006 Mar 27. Hum Reprod Update. 2006. PMID: 16567347 Review.
Cited by
-
Reproductive outcomes in fresh transfer cycles and antagonists with premature luteinizing and/or progesterone surge: a single center retrospective cohort study.Front Endocrinol (Lausanne). 2024 Sep 24;15:1411106. doi: 10.3389/fendo.2024.1411106. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39381441 Free PMC article.
-
Evaluation of gonadotropin-releasing hormone agonist and antagonist protocols on pregnancy outcomes in POSEIDON groups 3 and 4: A randomized controlled trial.Int J Reprod Biomed. 2025 Jun 10;23(3):241-250. doi: 10.18502/ijrm.v23i3.18775. eCollection 2025 Mar. Int J Reprod Biomed. 2025. PMID: 40567909 Free PMC article.
-
Impact of growth hormone on IVF/ICSI outcomes and endometrial receptivity of patients undergoing GnRH antagonist protocol with fresh embryo transfer: a pilot study.Front Endocrinol (Lausanne). 2023 Aug 31;14:1225121. doi: 10.3389/fendo.2023.1225121. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37727454 Free PMC article.
References
-
- Kolibianakis EM, Venetis CA, Kalogeropoulou L, Papanikolaou E, Tarlatzis BC. Fixed versus flexible gonadotropin-releasing hormone antagonist administration in in vitro fertilization: a randomized controlled trial. Fertil Steril. 2011;95(2):558–562. doi: 10.1016/j.fertnstert.2010.05.052. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources