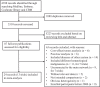
Efficacy of hematopoietic stem cell mobilization regimens in patients with hematological malignancies: a systematic review and network meta-analysis of randomized controlled trials
- PMID: 35317856
- PMCID: PMC8939102
- DOI: 10.1186/s13287-022-02802-6
Efficacy of hematopoietic stem cell mobilization regimens in patients with hematological malignancies: a systematic review and network meta-analysis of randomized controlled trials
Abstract
Background: Efficient mobilization of hematopoietic stem cells (HSCs) from bone marrow niche into circulation is the key to successful collection and transplantation in patients with hematological malignancies. The efficacy of various HSCs mobilization regimens has been widely investigated, but the results are inconsistent.
Methods: We performed comprehensive databases searching for eligible randomized controlled trials (RCTs) that comparing the efficacy of HSCs mobilization regimens in patients with hematological malignancies. Bayesian network meta-analyses were performed with WinBUGS. Standard dose of granulocyte colony-stimulating factor (G-CSF SD) was chosen as the common comparator. Estimates of relative treatment effects for other regimens were reported as mean differences (MD) or odds ratio (OR) with associated 95% credibility interval (95% CrI). The surface under the cumulative ranking curve (SUCRA) were obtained to present rank probabilities of all included regimens.
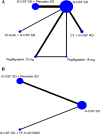
Results: Databases searching and study selection identified 44 eligible RCTs, of which the mobilization results are summarized. Then we compared the efficacy of mobilization regimens separately for patients with multiple myeloma (MM) and non-Hodgkin lymphoma (NHL) by including 13 eligible trials for network meta-analysis, involving 638 patients with MM and 592 patients with NHL. For patients with MM, data are pooled from 8 trials for 6 regimens, including G-CSF in standard dose (SD) or reduced dose (RD) combined with cyclophosphamide (CY), intermediate-dose cytarabine (ID-AraC) or plerixafor. The results show that compared with G-CSF SD alone, 3 regimens including ID-AraC + G-CSF SD (MD 14.29, 95% CrI 9.99-18.53; SUCRA 1.00), G-CSF SD + Plerixafor SD (MD 4.15, 95% CrI 2.92-5.39; SUCRA 0.80), and CY + G-CSF RD (MD 1.18, 95% CrI 0.29-2.07; SUCRA 0.60) are associated with significantly increased total number of collected CD34+ cells (× 106/kg), among which ID-AraC + G-CSF SD ranked first with a probability of being best regimen of 100%. Moreover, ID-AraC + G-CSF SD and G-CSF SD + Plerixafor SD are associated with significantly higher successful rate of achieving optimal target (collecting ≥ 4-6 × 106 CD34+ cells/kg). For patients with NHL, data are pooled from 5 trials for 4 regimens, the results show that compared with G-CSF SD alone, G-CSF SD + Plerixafor SD (MD 3.62, 95% CrI 2.86-4.38; SUCRA 0.81) and G-CSF SD plus the new CXC chemokine receptor-4 (CXCR-4) antagonist YF-H-2015005 (MD 3.43, 95% CrI 2.51-4.35; SUCRA 0.69) are associated with significantly higher number of total CD34+ cells collected. These 2 regimens are also associated with significantly higher successful rate of achieving optimal target. There are no significant differences in rate of achieving optimal target between G-CSF SD + Plerixafor SD and G-CSF + YF-H-2015005.
Conclusions: In conclusion, ID-AraC plus G-CSF is associated with the highest probability of being best mobilization regimen in patients with MM. For patients with NHL, G-CSF in combination with plerixafor or YF-H-2015005 showed similar improvements in HSCs mobilization efficacy. The relative effects of other chemotherapy-based mobilization regimens still require to be determined with further investigations.
Keywords: Cyclophosphamide; G-CSF; Hematological malignancies; Hematopoietic stem cell mobilization; Plerixafor.
© 2022. The Author(s).
Conflict of interest statement
The authors declared no conflicts of interest.
Figures


Similar articles
-
Comparison of the efficacy of hematopoietic stem cell mobilization regimens: a systematic review and network meta-analysis of preclinical studies.Stem Cell Res Ther. 2021 May 29;12(1):310. doi: 10.1186/s13287-021-02379-6. Stem Cell Res Ther. 2021. PMID: 34051862 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Moving Beyond G-CSF Mobilization-Learning From a 15-Year Experience of Different Stem Cell Mobilization Regimens in Multiple Myeloma.Cancer Med. 2025 Jul;14(14):e71068. doi: 10.1002/cam4.71068. Cancer Med. 2025. PMID: 40667650 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
Cited by
-
The Progress of Autologous Hematopoietic Stem Cell Transplantation in the Treatment of Multiple Myeloma (Review).Technol Cancer Res Treat. 2025 Jan-Dec;24:15330338251321349. doi: 10.1177/15330338251321349. Epub 2025 Mar 25. Technol Cancer Res Treat. 2025. PMID: 40129396 Free PMC article. Review.
-
Efficacy and safety of CD22-specific and CD19/CD22-bispecific CAR-T cell therapy in patients with hematologic malignancies: A systematic review and meta-analysis.Front Oncol. 2022 Dec 29;12:954345. doi: 10.3389/fonc.2022.954345. eCollection 2022. Front Oncol. 2022. PMID: 36644638 Free PMC article.
-
Evaluation of efficacy and safety in the use of cytarabine for mobilization of hematopoietic stem cells in a reference hospital in northeastern Brazil.Hematol Transfus Cell Ther. 2024 Oct-Dec;46(4):428-433. doi: 10.1016/j.htct.2023.08.007. Epub 2023 Oct 29. Hematol Transfus Cell Ther. 2024. PMID: 37951835 Free PMC article.
-
Stem Cell Mobilization with Ixazomib and G-CSF in Patients with Multiple Myeloma.Cancers (Basel). 2023 Jan 9;15(2):430. doi: 10.3390/cancers15020430. Cancers (Basel). 2023. PMID: 36672379 Free PMC article.
-
Antiretrovirals to CCR5 CRISPR/Cas9 gene editing - A paradigm shift chasing an HIV cure.Clin Immunol. 2023 Oct;255:109741. doi: 10.1016/j.clim.2023.109741. Epub 2023 Aug 21. Clin Immunol. 2023. PMID: 37611838 Free PMC article. Review.
References
-
- Chiappella A, Martelli M, Angelucci E, Brusamolino E, Evangelista A, Carella AM, et al. Rituximab-dose-dense chemotherapy with or without high-dose chemotherapy plus autologous stem-cell transplantation in high-risk diffuse large B-cell lymphoma (DLCL04): final results of a multicentre, open-label, randomised, controlled, phase 3 study. Lancet Oncol. 2017;18(8):1076–1088. - PubMed
-
- Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet. 2002;359(9323):2065–2071. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

