A novel and efficient CD22 CAR-T therapy induced a robust antitumor effect in relapsed/refractory leukemia patients when combined with CD19 CAR-T treatment as a sequential therapy
- PMID: 35317863
- PMCID: PMC8939233
- DOI: 10.1186/s40164-022-00270-5
A novel and efficient CD22 CAR-T therapy induced a robust antitumor effect in relapsed/refractory leukemia patients when combined with CD19 CAR-T treatment as a sequential therapy
Abstract
Background: CD19 chimeric antigen receptor (CAR) therapy has achieved impressive success in relapsed or refractory (R/R) B-cell malignancies, but relapse due to antigen escape is increasingly appearing reported. As the expression profile of CD22 is similar to that of CD19, CD22 has become a candidate target when CD19 CAR-T therapy fails.
Methods: A novel CD22 CAR incorporating scFv derived from an HIB22 hybridoma which bound the first and second Ig-like extracellular domains of CD22 antigen was constructed. Preclinical investigation of the CD22 CAR-T therapy against B-cell malignancies was evaluated by coculturing CD22 CAR-T cells with tumor cell lines or primary blasts from patients in vitro and using a xenograft mouse model in vivo. Further clinical study of CD22/CD19 CAR-T sequential therapy was conducted in 4 R/R adult B-cell acute lymphoblastic leukemia (B-ALL) patients.
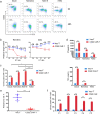
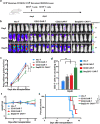
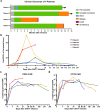
Results: The novel CD22 CAR-T treatment had specific cytotoxicity to CD22 + target cells, and the survival time of mice in the CD22 CAR-T treatment group was significantly prolonged. Furthermore, it's validated that sequential CD22/CD19 CAR-T therapy was significantly superior than single CD19 or CD22 CAR-T treatment in a relapse xenograft model. All 4 patients achieved complete remission (CR) with negative minimal residual disease (MRD), including 3 patients who had received prior CD19-related immunotherapy. The proliferation of CD19 and CD22 CAR-T cells was observed respectively in vivo, and 3 of the 4 patients experienced cytokine release syndrome (CRS); 2 of these patients had grade 1 CRS and 1 had grade 3 CRS. Long term follow-up showed that 3 of the 4 (75%) patients had sustained CR for up to 1 year. Analysis of antigen expression in the relapsed patients demonstrated that loss or diminution of CD19 and CD22 expression might cause antigen escape from CAR-T surveillance.
Conclusions: In summary, the novel CD22 CAR-T therapy was validated with antitumor effects both in vitro and in vivo. Furthermore, our study demonstrated the safety and robust efficacy of sequential CD22/CD19 CAR-T therapy in xenograft models and clinical trials, especially as the salvage treatment for R/R B-ALL patients with antigen loss or in whom anti-CD19 related immunotherapy failure failed.
Trial registration: Chinese Clinical Trial Registry (ChiCTR): ChiCTR1900025419, Supplementarily registered 26 August, 2019.
Keywords: Antigen escape; B-ALL; CAR-T; CD19; CD22; HIB22; Sequential therapy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




Similar articles
-
Sequential CD19 and CD22 chimeric antigen receptor T-cell therapy for childhood refractory or relapsed B-cell acute lymphocytic leukaemia: a single-arm, phase 2 study.Lancet Oncol. 2023 Nov;24(11):1229-1241. doi: 10.1016/S1470-2045(23)00436-9. Epub 2023 Oct 17. Lancet Oncol. 2023. PMID: 37863088 Clinical Trial.
-
Safety and efficacy of co-administration of CD19 and CD22 CAR-T cells in children with B-ALL relapse after CD19 CAR-T therapy.J Transl Med. 2023 Mar 22;21(1):213. doi: 10.1186/s12967-023-04019-4. J Transl Med. 2023. PMID: 36949487 Free PMC article.
-
Anti-CD22 CAR-T Cell Therapy as a Salvage Treatment in B Cell Malignancies Refractory or Relapsed After Anti-CD19 CAR-T therapy.Onco Targets Ther. 2021 Jul 2;14:4023-4037. doi: 10.2147/OTT.S312904. eCollection 2021. Onco Targets Ther. 2021. PMID: 34239307 Free PMC article.
-
Differences in efficacy and safety among CAR-Ts anti-CD19/CD22, anti-CD19, and anti-CD22, in adult patients with relapse/refractory B-cell acute lymphoblastic leukemia: a meta-analysis and systematic review.Leuk Lymphoma. 2023 Nov-Dec;64(11):1822-1831. doi: 10.1080/10428194.2023.2243357. Epub 2023 Aug 7. Leuk Lymphoma. 2023. PMID: 37548560
-
Efficacy and safety of CD19 combined with CD22 or CD20 chimeric antigen receptor T-cell therapy for hematological malignancies.Front Immunol. 2025 May 13;16:1577360. doi: 10.3389/fimmu.2025.1577360. eCollection 2025. Front Immunol. 2025. PMID: 40433368 Free PMC article.
Cited by
-
Effectiveness and safety of CD22 and CD19 dual-targeting chimeric antigen receptor T-cell therapy in patients with relapsed or refractory B-cell malignancies: A meta-analysis.Cancer Med. 2023 Sep;12(18):18767-18785. doi: 10.1002/cam4.6497. Epub 2023 Sep 5. Cancer Med. 2023. PMID: 37667978 Free PMC article.
-
Multidisciplinary recommendations for the management of CAR-T recipients in the post-COVID-19 pandemic era.Exp Hematol Oncol. 2023 Jul 27;12(1):66. doi: 10.1186/s40164-023-00426-x. Exp Hematol Oncol. 2023. PMID: 37501090 Free PMC article. Review.
-
The evolution of acute lymphoblastic leukemia research and therapy at MD Anderson over four decades.J Hematol Oncol. 2023 Mar 16;16(1):22. doi: 10.1186/s13045-023-01409-5. J Hematol Oncol. 2023. PMID: 36927623 Free PMC article. Review.
-
Strategies for Reducing Toxicity and Enhancing Efficacy of Chimeric Antigen Receptor T Cell Therapy in Hematological Malignancies.Int J Mol Sci. 2023 May 23;24(11):9115. doi: 10.3390/ijms24119115. Int J Mol Sci. 2023. PMID: 37298069 Free PMC article. Review.
-
Targeting Interleukin-13 Receptor α2 and EphA2 in Aggressive Breast Cancer Subtypes with Special References to Chimeric Antigen Receptor T-Cell Therapy.Int J Mol Sci. 2024 Mar 28;25(7):3780. doi: 10.3390/ijms25073780. Int J Mol Sci. 2024. PMID: 38612592 Free PMC article. Review.
References
-
- Singh AK, McGuirk JP. CAR T cells: continuation in a revolution of immunotherapy. Lancet Oncol. 2020;21(3):e168–e178. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

